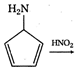
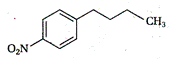
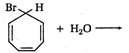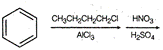question_answer 1) Two particles of mass \[{{m}_{1}}\]and\[{{m}_{2}};\] approach each other due to their mutual gravitational attraction only. Then,
A)
accelerations of both the particles are equal
done
clear
B)
acceleration of the particle of mass \[{{m}_{1}}\]a is proportional to \[{{m}_{1}}\]
done
clear
C)
acceleration of the particle of mass \[{{m}_{1}}\]is proportional to \[{{m}_{2}}\]
done
clear
D)
acceleration of the particle of mass \[{{m}_{1}}\]is inversely\[{{m}_{1}}\] proportional to\[{{m}_{1}}\]
done
clear
View Answer play_arrow
question_answer 2) Three bodies of the same material and having masses \[m,m\]and 3m are at temperatures \[40{{\,}^{o}}C,\,\,50{{\,}^{o}}C\]and \[60{{\,}^{o}}C,\] respectively. If the bodies are brought in thermal contact, the final temperature will be
A)
\[45{{\,}^{o}}C\]
done
clear
B)
\[54{{\,}^{o}}C\]
done
clear
C)
\[52{{\,}^{o}}C\]
done
clear
D)
\[48{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 3) A satellite has kinetic energy K, potential energy V and total energy E. Which of the following statements is true?
A)
\[K=-V/2\]
done
clear
B)
\[K=V/2\]
done
clear
C)
\[E=K/2\]
done
clear
D)
\[E=-K/2\]
done
clear
View Answer play_arrow
question_answer 4)
An object is located 4 m from the first of two thin converging lenses of focal lengths 2 m and 1 m, respectively. The lenses are separated by 3m. The final image formed by the second lens is located from the source at a distance of
A)
8.0m
done
clear
B)
5.5m
done
clear
C)
6.0 m
done
clear
D)
6.5 m
done
clear
View Answer play_arrow
question_answer 5) A simple pendulum of length L swings in a vertical plane. The tension of the string when it makes an angle \[\theta \] with the vertical and the bob of mass m moves with a speed v is (g is the gravitational acceleration)
A)
\[m{{v}^{2}}/L\]
done
clear
B)
\[mg\,\cos \theta +m{{v}^{2}}/L\]
done
clear
C)
\[mg\,\cos \theta \,-m{{v}^{2}}/L\]
done
clear
D)
\[mg\,\cos \theta \]
done
clear
View Answer play_arrow
question_answer 6) The length of a metal wire is \[{{L}_{1}}\]when the tension is \[{{T}_{1}}\]and \[{{L}_{2}}\]when the tension is \[{{T}_{2}}.\]The unstretched length of wire is
A)
\[\frac{{{L}_{1}}+{{L}_{2}}}{2}\]
done
clear
B)
\[\sqrt{{{L}_{1}}{{L}_{2}}}\]
done
clear
C)
\[\frac{{{T}_{2}}{{L}_{1}}-{{T}_{1}}{{L}_{2}}}{{{T}_{2}}-{{T}_{1}}}\]
done
clear
D)
\[\frac{{{T}_{2}}{{L}_{1}}+{{T}_{1}}{{L}_{2}}}{{{T}_{2}}+{{T}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 7) The line AA is on charged infinite A conducting plane which is perpendicular to the plane of the paper. The plane has a surface density of charge \[\sigma \] and B is hall of mass m with a like charge of magnitude q. B is connected by string from a point on the line AA. The tangent of angle \[(\theta )\] formed between the line AA and tile string is
A)
\[\frac{q\sigma }{2{{\varepsilon }_{0}}mg}\]
done
clear
B)
\[\frac{q\sigma }{4\pi {{\varepsilon }_{0}}mg}\]
done
clear
C)
\[\frac{q\sigma }{2\pi {{\varepsilon }_{0}}mg}\]
done
clear
D)
\[\frac{q\sigma }{{{\varepsilon }_{0}}mg}\]
done
clear
View Answer play_arrow
question_answer 8)
The current I is in the circuit shown is
A)
1.33 A
done
clear
B)
zero
done
clear
C)
2.00 A
done
clear
D)
1.00 A
done
clear
View Answer play_arrow
question_answer 9) A hollow sphere of external radius R and thickness \[t(t<<R)\]is made of a metal of density p. The sphere will float in water, if
A)
\[t\le \frac{R}{\rho }\]
done
clear
B)
\[t\le \frac{R}{3\rho }\]
done
clear
C)
\[t\le \frac{R}{2\rho }\]
done
clear
D)
\[t\ge \frac{R}{3\rho }\]
done
clear
View Answer play_arrow
question_answer 10) A metal wire of circular cross-section has a resistance \[{{R}_{1}}.\]The wire is now stretched without breaking, so that its length is doubled and the density is assumed to remain the same. If the resistance of the wire now becomes \[{{R}_{2}},\]then \[{{R}_{2}}:{{R}_{1}}\]is
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
4:1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 11)
Assume that each diode as shown in the figure has a forward bias resistance of \[50\Omega \]and an infinite reverse bias resistance. The current through the resistance \[150\,\Omega \]is
A)
0.66 A
done
clear
B)
0.05 A
done
clear
C)
zero
done
clear
D)
0.04 A
done
clear
View Answer play_arrow
question_answer 12) The rms speed of oxygen is v at a particular temperature. If the temperature is doubled and oxygen molecules dissociate into oxygen atoms, the rms speed becomes
A)
\[v\]
done
clear
B)
\[\sqrt{2}v\]
done
clear
C)
\[2v\]
done
clear
D)
\[4v\]
done
clear
View Answer play_arrow
question_answer 13) Two particles, A and B, having equal charges, after being accelerated through the same potential difference enter into a region of uniform magnetic field and the particles describe circular paths of radii \[{{R}_{1}}\]and\[{{R}_{2}},\] respectively. The ratio of the masses of A and B is
A)
\[\sqrt{{{R}_{1}}/{{R}_{2}}}\]
done
clear
B)
\[{{R}_{1}}/{{R}_{2}}\]
done
clear
C)
\[{{({{R}_{1}}/{{R}_{2}})}^{2}}\]
done
clear
D)
\[{{({{R}_{2}}/{{R}_{1}})}^{2}}\]
done
clear
View Answer play_arrow
question_answer 14) A large number of particles are placed around the origin, each at a distance R from the origin. The distance of the centre of mass of the system from the origin is
A)
equal to R
done
clear
B)
less than equal to R
done
clear
C)
greater than R
done
clear
D)
greater than equal to R
done
clear
View Answer play_arrow
question_answer 15) A straight conductor 0.1 m long moves in a uniform magnetic field 0.1 T. The velocity of the conductor is 15 m/s and is directed perpendicular to the field. The emf induced between the two ends of the conductor is
A)
0.10V
done
clear
B)
0.15V
done
clear
C)
1.50 V
done
clear
D)
15.00 V
done
clear
View Answer play_arrow
question_answer 16) A ray of light is incident at an angle \[i\]on a glass slab of refractive index \[\,\mu .\]The angle between reflected and refracted light is \[{{90}^{o}}.\] Then, the relationship between \[i\]and \[\,\mu .\]is
A)
\[i={{\tan }^{-1}}\left( \frac{1}{\mu } \right)\]
done
clear
B)
\[\tan \,i=\mu \]
done
clear
C)
\[\sin \,i=\mu \]
done
clear
D)
\[\cos \,i=\mu \]
done
clear
View Answer play_arrow
question_answer 17)
Two particles A and B are moving as shown in the figure.
A)
\[9.8\text{ }kg\text{ }{{m}^{2}}/s\]
done
clear
B)
zero
done
clear
C)
\[52.7\,kg\,{{m}^{2}}/s\]
done
clear
D)
\[37.9\,kg\,{{m}^{2}}/s\]
done
clear
View Answer play_arrow
question_answer 18) A 20 cm long capillary tube is dipped vertically in water and the liquid rises upto 10 cm. If the entire system is kept is a freely falling platform, the length of the water column in the tube will be
A)
5 cm
done
clear
B)
10 cm
done
clear
C)
15 cm
done
clear
D)
20 cm
done
clear
View Answer play_arrow
question_answer 19) A train is moving with a uniform speed of 33 m/s and an observer is approaching the train with the same speed. If the train blows a whistle of frequency 1000 Hz and the velocity of sound is 333 m/s, then the apparent frequency of the sound that the observer hears is
A)
1220 Hz
done
clear
B)
1099 Hz
done
clear
C)
1110 Hz
done
clear
D)
1200 Hz
done
clear
View Answer play_arrow
question_answer 20) A photon of wavelength 300 nm interacts with a stationary hydrogen atom in ground state. During the interaction, whole energy of the photon is transferred to the electron of the atom. State which possibility is correct. (Consider, Plank constant \[=4\times {{10}^{-15}}\,eVs,\] velocity of light \[=3\times {{10}^{8}}m/s,\] ionization energy of hydrogen\[=13.6\text{ }eV\])
A)
Electron will be knocked out of the atom
done
clear
B)
Electron will go to any excited state of the atom
done
clear
C)
Electron will go only to first excited state of the atom
done
clear
D)
Electron will keep orbiting in the ground state of the atom
done
clear
View Answer play_arrow
question_answer 21)
Particle A moves along X-axis with a uniform velocity of magnitude 10 m/s. Particle B moves with uniform velocity 20 m/s along a direction making an angle of \[{{60}^{o}}\] with the positive direction of X-axis as shown in the figure. The relative velocity of B with respect to that of A is
A)
10 m/s along X-axis
done
clear
B)
\[10\sqrt{3}\,m/s\]along Y-axis (perpendicular to X-axis)
done
clear
C)
\[10\sqrt{5}\,m/s\]along the bisection of the velocities of A and B
done
clear
D)
30 m/s along negative X-axis
done
clear
View Answer play_arrow
question_answer 22) When light is refracted from a surface, which of its following physical parameters does not change?
A)
Velocity
done
clear
B)
Amplitude
done
clear
C)
Frequency
done
clear
D)
Wavelength
done
clear
View Answer play_arrow
question_answer 23) A solid maintained at \[{{t}^{o}}_{1}C\]is kept in an evacuated chamber at temperature\[{{t}^{o}}_{2}C\]\[({{t}_{2}}>>{{t}_{1}}).\]The rate of heat absorbed by the body is proportional to
A)
\[t_{2}^{4}-t_{1}^{4}\]
done
clear
B)
\[(t_{2}^{4}+273)-(t_{1}^{4}+273)\]
done
clear
C)
\[{{t}_{2}}-{{t}_{1}}\]
done
clear
D)
\[t_{2}^{2}-t_{1}^{2}\]
done
clear
View Answer play_arrow
question_answer 24)
Block B lying on a table weighs W. The coefficient of static friction between the block and the table is \[\mu .\]Assume that the cord between B and the knot is horizontal. The maximum weight of the block A for which the system will be stationary is
A)
\[\frac{W\tan \theta }{\mu }\]
done
clear
B)
\[\mu W\,\tan \theta \]
done
clear
C)
\[\mu W\sqrt{1+{{\tan }^{2}}\theta }\]
done
clear
D)
\[\mu W\sin \theta \]
done
clear
View Answer play_arrow
question_answer 25)
The inputs to the digital circuit are as below. The output Y is
A)
\[A+B+\bar{C}\]
done
clear
B)
\[(A+B)\bar{C}\]
done
clear
C)
\[\bar{A}+\bar{B}+\bar{C}\]
done
clear
D)
\[\bar{A}+\bar{B}+C\]
done
clear
View Answer play_arrow
question_answer 26) Two particles A and B having different masses are projected from a tower with same speed. A is projected vertically upward B vertically downward. On reaching the ground
A)
velocity of A is greater than that of B
done
clear
B)
velocity of B is greater than that of A
done
clear
C)
both A and B attain the same velocity
done
clear
D)
the particle with the larger mass attains velocity
done
clear
View Answer play_arrow
question_answer 27) The work function of metals is in the range of 2eV to 5eV. Find which of the following wavelength of light cannot be used for photoelectric effect? (Consider, Plank constant \[=4\times {{10}^{-15}}\,eVs,\] velocity of light\[=3\times {{10}^{8}}\,m/s\])
A)
510 nm
done
clear
B)
650 nm
done
clear
C)
400 nm
done
clear
D)
570 nm
done
clear
View Answer play_arrow
question_answer 28) A thin plastic sheet of refractive index used to cover one of the slits of a double arrangement. The central point on the screen is now occupied by what would have been the 7th bright fringe before the plastic was used. If the wavelength of light is 600 nm, what is the thickness (in \[\mu m\]) of the plastic sheet
A)
7
done
clear
B)
4
done
clear
C)
8
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 29) The length of an open organ pipe is twice the length of another closed organ pipe. The fundamental frequency of the open pipe is 100 Hz. The frequency of the third harmonic of the closed pipe is
A)
100 Hz
done
clear
B)
200 Hz
done
clear
C)
300 Hz
done
clear
D)
150 Hz
done
clear
View Answer play_arrow
question_answer 30) A \[5\,\mu F\]capacitor is connected in series with a \[10\,\mu F\]capacitor. When a 300 V potential difference is applied across this combination/the total energy stored in the capacitors is
A)
15 J
done
clear
B)
1.5 J
done
clear
C)
0.15 J
done
clear
D)
0.10 J
done
clear
View Answer play_arrow
question_answer 31)
A cylinder of height A is filled with water and is kept on a block of height \[h/2.\]The level of water in the cylinder is kept constant. Four holes numbered 1, 2, 3 and 4 are at the side of the cylinder and at heights \[0,h/4,h/2\] and \[3h/4,\] respectively. When all four holes are opened together, the hole from which water will reach farthest distance on the plane PQ is the whole number.
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 32) The pressure p, volume V and temperature T for a certain gas are related by \[p=\frac{AT-B{{T}^{2}}}{V},\] where A and B are constants. The work done by the gas when the temperature changes from \[{{T}_{1}}\]to \[{{T}_{2}}\]while the pressure remains constant, is given by
A)
\[A({{T}_{2}}-{{T}_{1}})+B(T_{2}^{2}-T_{1}^{2})\]
done
clear
B)
\[\frac{A({{T}_{2}}-{{T}_{1}})}{{{V}_{2}}-{{V}_{1}}}-\frac{B(T_{2}^{2}-T_{1}^{2})}{{{V}_{2}}-{{V}_{1}}}\]
done
clear
C)
\[A({{T}_{2}}-{{T}_{1}})-\frac{B}{2}(T_{2}^{2}-T_{1}^{2})\]
done
clear
D)
\[\frac{A({{T}_{2}}-T_{1}^{2})}{{{V}_{2}}-{{V}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 33)
In the circuit shown below, the switch is kept in position a for a long time and is then thrown to position b. The amplitude of the resulting oscillating current is given by
A)
\[E\sqrt{L/C}\]
done
clear
B)
\[E/R\]
done
clear
C)
infinity
done
clear
D)
\[E\sqrt{C/L}\]
done
clear
View Answer play_arrow
question_answer 34) A charge q is placed at one corner of a cube. The electric flux through any of the three faces adjacent to the charge is zero. The flux through any one of the other three faces is
A)
\[q/3\,{{\varepsilon }_{0}}\]
done
clear
B)
\[q/6\,{{\varepsilon }_{0}}\]
done
clear
C)
\[q/12\,{{\varepsilon }_{0}}\]
done
clear
D)
\[q/24\,{{\varepsilon }_{0}}\]
done
clear
View Answer play_arrow
question_answer 35)
Two cells A and B of emf 2V and 1.5V respectively, are connected as shown in figure through an external resistance \[10\,\Omega .\] The internal resistance of each cell is \[5\,\Omega .\]The potential difference \[{{E}_{A}}\]and \[{{E}_{B}}\]across the terminals of the cells A and B respectively are
A)
\[{{E}_{A}}=2.0V,{{E}_{B}}=1.5\,V\]
done
clear
B)
\[{{E}_{A}}=2.125\,V,\,{{E}_{B}}=1.375\,V\]
done
clear
C)
\[{{E}_{A}}=1.875\,V,{{E}_{B}}=1.625\,V\]
done
clear
D)
\[{{E}_{A}}=1.875\,V,{{E}_{B}}=1.375V\]
done
clear
View Answer play_arrow
question_answer 36) Two charges \[+\text{ }q\]and \[-\text{ }q\]are placed at a distance a in a uniform electric field. The dipole moment of the combination is \[2qa(cos\,\theta \hat{i}+\sin \theta \hat{j}),\] where, \[\theta \]is the angle between the direction of the field and the line joining the two charges. Which of the following statement(s) is/are correct?
A)
The torque exerted by the field on the dipole vanishes
done
clear
B)
The net force on the dipole vanishes
done
clear
C)
The torque is independent of the choice of coordinates
done
clear
D)
The net force is independent of a
done
clear
View Answer play_arrow
question_answer 37) Find the right condition(s) for Fraunhoffer diffraction due to a single slit.
A)
Source is at infinite distance and the incident beam has converged at the slit
done
clear
B)
Source is near to the slit and the incident beam is parallel
done
clear
C)
Source is at infinity and the incident beam is parallel
done
clear
D)
Source is near to the slit and the incident beam has converged at the slit
done
clear
View Answer play_arrow
question_answer 38) The conducting loop in the form of a circle is placed in a uniform magnetic field with its plane perpendicular to the direction of the field. An emf will be induced in the loop, if
A)
it is translated parallel to itself
done
clear
B)
it is rotated about one of its diameters
done
clear
C)
it is rotated about its own axis which is parallel to the field
done
clear
D)
the loop is deformed from the original shape
done
clear
View Answer play_arrow
question_answer 39) A circular disc rolls on a horizontal floor without slipping and the centre of the disc moves with a uniform velocity v. Which of the following values of the velocity at a point on the rim of the disc can have?
A)
\[v\]
done
clear
B)
\[-\text{ }v\]
done
clear
C)
\[2v\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 40) Consider two particles of different masses. In which of the following situations the heavier of the two particles will have smaller de-Broglie wavelength?
A)
Both have a free fall through the same height
done
clear
B)
Both move with the same kinetic energy
done
clear
C)
Both move with the same linear momentum
done
clear
D)
Both move with the same speed
done
clear
View Answer play_arrow
question_answer 41)
Match the flame colours of the alkaline earth metal salts in the Bunsen burner. A. Calcium P. Brick red B. Strontium q. Apple green C. Barium r. Crimson
A)
A-p B-r C-q
done
clear
B)
A-r B-p C-q
done
clear
C)
A-q B-r C-p
done
clear
D)
A-p B-q C-r
done
clear
View Answer play_arrow
question_answer 42) Extraction of gold (Au) involves the formation of complex ions X and Y. Gold ore \[\xrightarrow[C{{N}^{-}}.{{H}_{2}}O.{{O}_{2}}]{Roasting}H{{O}^{-}}+X\xrightarrow{Zn}Y+Au\] X and y respectively are
A)
\[Au(CN)_{2}^{-}\]and \[Zn(CN)_{4}^{2-}\]
done
clear
B)
\[Au(CN)_{4}^{3-}\]and\[Zn(CN)_{4}^{2-}\]
done
clear
C)
\[Au(CN)_{3}^{-}\]and \[Zn(CN)_{6}^{4-}\]
done
clear
D)
\[Au(CN)_{4}^{-}\] and \[Zn(CN)_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 43) The atomic number of cerium (Ce) is 58. The correct electronic configuration of \[C{{e}^{3+}}\]ion is
A)
\[[Xe]4{{f}^{1}}\]
done
clear
B)
\[[Kr]4{{f}^{1}}\]
done
clear
C)
\[[Xe]4{{f}^{13}}\]
done
clear
D)
\[[Kr]4{{d}^{1}}\]
done
clear
View Answer play_arrow
question_answer 44)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 45)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 46) Sulphuryl chloride \[(S{{O}_{2}}C{{l}_{2}})\]reacts with white phosphorus \[({{P}_{4}})\] to give
A)
\[PC{{l}_{5}},S{{O}_{2}}\]
done
clear
B)
\[OPC{{l}_{3}},SOC{{l}_{2}}\]
done
clear
C)
\[PC{{l}_{5}},S{{O}_{2}},{{S}_{2}}C{{l}_{2}}\]
done
clear
D)
\[OPC{{l}_{3}},S{{O}_{2}},{{S}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 47) The number of lone pairs of electrons on the central atoms of \[{{\text{H}}_{\text{2}}}\text{O,SnC}{{\text{l}}_{\text{2}}}\text{,PC}{{\text{l}}_{\text{3}}}\]respectively, are
A)
2,1, 1,3
done
clear
B)
2,2, 1,3
done
clear
C)
3, 1, 1, 2
done
clear
D)
2,1, 2, 3
done
clear
View Answer play_arrow
question_answer 48) Consider the following salts: \[NaCl,HgC{{l}_{2}},\text{ }H{{g}_{2}}C{{l}_{2}},\text{ }CuC{{l}_{2}},\text{ }CuCl\]and \[\text{AgCl}\text{.}\]Identify the correct set of insoluble salts in water.
A)
\[H{{g}_{2}}C{{l}_{2}},\text{ }CuCl,\text{ }AgCl\]
done
clear
B)
\[~HgC{{l}_{2}}\text{, }CuCl,\text{ }AgCl\]
done
clear
C)
\[~H{{g}_{2}}C{{l}_{2}}\text{, }CuC{{l}_{2}},\text{ }AgCl\]
done
clear
D)
\[~H{{g}_{2}}C{{l}_{2}},\text{ }CuCl,\text{ }NaCl\]
done
clear
View Answer play_arrow
question_answer 49) In the following compound, the number of sp-hybridised carbons are \[C{{H}_{2}}=C=\underset{CN}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C\equiv CH\]
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 50) For the reaction \[A+2B\xrightarrow{{}}C,\]the reaction rate is doubled, if the concentration of A is doubled. The rate is increased by four times when concentrations of both A and B are increased by four times. The order of the reaction is
A)
3
done
clear
B)
0
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 51) At a certain temperature, the value of the slope of the plot of osmotic pressure [n) against concentration (C in\[\text{mol}\,{{\text{L}}^{-1}}\]) of a certain polymer solution is 291R. The temperature at which osmotic pressure is measured, is (R is gas constant)
A)
\[271{{\,}^{o}}C\]
done
clear
B)
\[18{{\,}^{o}}C\]
done
clear
C)
564 K
done
clear
D)
18 K
done
clear
View Answer play_arrow
question_answer 52) The RMS velocity of CO gas molecules at \[27{{\,}^{o}}C\]is approximately 1000 m/s. For \[{{N}_{2}}\]molecules at 600 K, the RMS velocity is approximately
A)
2000 m/s
done
clear
B)
1414 m/s
done
clear
C)
1000 m/s
done
clear
D)
1500 m/s
done
clear
View Answer play_arrow
question_answer 53) A gas can be liquefied at temperature T and pressure p provided
A)
\[T={{T}_{c}}\]and \[p<{{p}_{c}}\]
done
clear
B)
\[T<{{T}_{c}}\]and \[p>{{p}_{c}}\]
done
clear
C)
\[T>{{T}_{c}}\]and \[p>{{p}_{c}}\]
done
clear
D)
\[T>{{T}_{c}}\]and \[p<{{p}_{c}}\]
done
clear
View Answer play_arrow
question_answer 54) The dispersed phase and dispersion medium of fog respectively are
A)
solid, liquid
done
clear
B)
liquid, liquid
done
clear
C)
liquid, gas
done
clear
D)
gas, liquid
done
clear
View Answer play_arrow
question_answer 55) The decreasing order of basic character of \[{{K}_{2}}O,\text{ }BaO,\text{ }CaO\]and \[MgO\]is
A)
\[{{K}_{2}}O>BaO>CaO>MgO\]
done
clear
B)
\[~{{K}_{2}}\text{O}>CaO>BaO>MgO\]
done
clear
C)
\[~~MgO>BaO>CaO>{{K}_{2}}O\]
done
clear
D)
\[~MgO>CaO>BaO>{{K}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 56) In aqueous alkaline solution, two electrons reduction of \[HO_{2}^{-}\]gives
A)
\[H{{O}^{-}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[O_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 57) Cold ferrous sulphate solution on absorption of NO develops brown colour due to the formation of
A)
paramagnetic \[[Fe{{({{H}_{2}}O)}_{5}}(NO)]S{{O}_{4}}\]
done
clear
B)
diamagnetic \[[Fe{{({{H}_{2}}O)}_{5}}({{N}_{3}})]S{{O}_{4}}\]
done
clear
C)
paramagnetic \[[Fe{{({{H}_{2}}O)}_{5}}(N{{O}_{3}})]{{[S{{O}_{4}}]}_{2}}\]
done
clear
D)
diamagnetic \[[Fe{{({{H}_{2}}O)}_{4}}(S{{O}_{4}})]N{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 58) Among Be, B, Mg and Al, the second ionisation potential is maximum for
A)
B
done
clear
B)
Be
done
clear
C)
Mg
done
clear
D)
Al
done
clear
View Answer play_arrow
question_answer 59) In a mixture, two enantiomers are found to be present in 85% and 15% respectively. The enantiomeric excess (ee) is
A)
85%
done
clear
B)
15%
done
clear
C)
70%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 60) 1,4-dimethylbenzene on heating with anhydrous \[\text{AlC}{{\text{l}}_{\text{3}}}\]and \[\text{HCl}\]produces
A)
1,2-dimethylbenzene
done
clear
B)
1,3-dimethylbenzene
done
clear
C)
1, 2. 3-trimethylbenzene
done
clear
D)
ethylbenzene
done
clear
View Answer play_arrow
question_answer 61)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 62) Suppose the mass of a single Ag-atom is \[m.\] Ag metal crystallises in fee lattice with unit cell of length q. The density of Ag metal in terms of a and \[m\] is
A)
\[\frac{4m}{{{a}^{3}}}\]
done
clear
B)
\[\frac{2m}{{{a}^{3}}}\]
done
clear
C)
\[\frac{m}{{{a}^{3}}}\]
done
clear
D)
\[\frac{m}{4{{a}^{3}}}\]
done
clear
View Answer play_arrow
question_answer 63) For the reaction, \[2S{{O}_{2}}(g)+{{O}_{2}}(g)2S{{O}_{3}}(g)\]at 300 K, the value of \[\Delta {{G}^{o}}\]is\[~-690.9\text{ }R.\] The equilibrium constant value for the reaction at that temperature is (R is gas constant)
A)
\[10\,\text{at}{{\text{m}}^{-1}}\]
done
clear
B)
\[10\,\text{atm}\]
done
clear
C)
10
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 64) At a particular temperature, the ratio of equivalent conductance to specific conductance of a 0.01 N NaCl solution is
A)
\[10{{\,}^{5}}c{{m}^{3}}\]
done
clear
B)
\[10{{\,}^{3}}c{{m}^{3}}\]
done
clear
C)
\[10\,c{{m}^{3}}\]
done
clear
D)
\[10{{\,}^{5}}c{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 65) The units of surface tension and viscosity of liquids respectively are
A)
\[kg\,{{m}^{-1}}{{s}^{-1}},N{{m}^{-1}}\]
done
clear
B)
\[kg\,{{s}^{-2}},kg\,{{m}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[N{{m}^{-1}},kg\,{{m}^{-1}}{{s}^{-2}}\]
done
clear
D)
\[kg\,{{s}^{-1}},\,kg\,{{m}^{-2}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 66) The ratio of volumes of \[\text{C}{{\text{H}}_{\text{3}}}\text{COOH}\,\text{0}\text{.1}\,\text{(N)}\]to \[\text{C}{{\text{H}}_{\text{3}}}\text{COONa}\,\,\text{0}\text{.1(N)}\]required to prepare a buffer solution of pH 5.74 is (Given \[p{{K}_{a}}\]of \[C{{H}_{3}}COOH\]is 4.74)
A)
10: 1
done
clear
B)
5:1
done
clear
C)
1 :5
done
clear
D)
1 : 10
done
clear
View Answer play_arrow
question_answer 67) The reaction of methyltrichloroacetate \[(C{{l}_{3}}CC{{O}_{2}}Me)\] with sodium methoxide \[(NaOMe)\] generates
A)
carbocation
done
clear
B)
carbine
done
clear
C)
carbanion
done
clear
D)
carbon radical
done
clear
View Answer play_arrow
question_answer 68) Best reagent for nuclear iodination of aromatic compounds is
A)
\[~KI/C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[{{I}_{2}}/C{{H}_{3}}CN\]
done
clear
C)
\[KI/C{{H}_{3}}COOH\]
done
clear
D)
\[{{I}_{2}}/HN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 69) In the Lassaignes test for the detection of nitrogen in an organic compound, the appearance of blue coloured compound is due to
A)
ferric ferricyanide
done
clear
B)
ferrous ferricyanide
done
clear
C)
ferric ferrocyanide
done
clear
D)
ferrous ferrocyanide
done
clear
View Answer play_arrow
question_answer 70) In the reaction \[R\,MgBr+HC{{(OEt)}_{3}}\xrightarrow{Ether,{{H}_{3}}{{O}^{+}}}P,\] the product P is
A)
\[RCHO\]
done
clear
B)
\[{{R}_{2}}CHOEt\]
done
clear
C)
\[{{R}_{3}}CH\]
done
clear
D)
\[RCH{{(OEt)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) Addition of sodium thiosulphate solution to a solution of silver nitrate gives X as white precipitate, insoluble in water but soluble in excess thiosulphate solution to give Y.. On boiling in water, Y gives Z. X, Y and Z respectively are
A)
\[A{{g}_{2}}{{S}_{2}}{{O}_{3}},N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}],A{{g}_{2}}S\]
done
clear
B)
\[A{{g}_{2}}S{{O}_{4}},Na[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}],A{{g}_{2}}{{S}_{2}}\]
done
clear
C)
\[A{{g}_{2}}{{S}_{2}}{{O}_{3}},N{{a}_{5}}[Ag{{({{S}_{2}}{{O}_{3}})}_{3}}],AgS\]
done
clear
D)
\[A{{g}_{2}}S{{O}_{3}},N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}],A{{g}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 72) At temperature of 298K, the emf of the following electrochemical cell, Ag \[(s)\,A{{g}^{+}}(0.1M)||Z{{n}^{2+}}(0.1M)|Zn(s)\]will be (Given\[{{E}^{o}}_{cell}=-1.562\,\text{V}\])
A)
\[-1.532V\]
done
clear
B)
\[-1.503V\]
done
clear
C)
\[1.532V\]
done
clear
D)
\[-3.06V\]
done
clear
View Answer play_arrow
question_answer 73) For the reaction,\[{{X}_{2}}{{Y}_{2}}(l)\xrightarrow{{}}2X{{Y}_{2}}(g)\]at \[300\,K,\] the values of \[\Delta U\]and\[\Delta S\] are 2 kcal and \[\text{20}\,\text{cal}\,{{\text{K}}^{-1}}\]respectively. The value of \[\Delta G\]for the reaction is
A)
\[~-3400\,\text{cal}\]
done
clear
B)
\[~3400\,\text{cal}\]
done
clear
C)
\[-2800\text{ cal}\]
done
clear
D)
\[2000\text{ cal}\]
done
clear
View Answer play_arrow
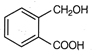
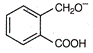
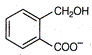
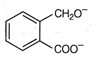
question_answer 74)
The total number of aromatic species generated in the following reactions is
A)
zero
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 75) Roasted copper pyrite on smelting with sand produces
A)
\[\text{FeSi}{{\text{O}}_{\text{3}}}\]as fusible slag and \[\text{C}{{\text{u}}_{\text{2}}}\text{S}\]as matte
done
clear
B)
\[\text{CaSi}{{\text{O}}_{3}}\]as infusible slag and \[\text{C}{{\text{u}}_{\text{2}}}\text{O}\]as matte
done
clear
C)
\[\text{C}{{\text{a}}_{3}}{{(P{{O}_{4}})}_{2}}\]as fusible slag and \[\text{C}{{\text{u}}_{\text{2}}}\text{S}\]as matte
done
clear
D)
\[F{{e}_{3}}{{(P{{O}_{4}})}_{2}}\]as infusible slag and \[\text{C}{{\text{u}}_{\text{2}}}\text{S}\]as matte
done
clear
View Answer play_arrow
question_answer 76) lonisation potential values of noble gases decrease down the group with increase in atomic size. Xenon forms binary fluorides by the direct reaction of elements. Identify the correct statement(s) from below.
A)
Only the heavier noble gases fo.rm such compounds
done
clear
B)
It happens because the noble gases have higher ionisation energies
done
clear
C)
It happens because the compounds are formed with electronegative ligands
done
clear
D)
Octet of electrons provide the stable arrangements
done
clear
View Answer play_arrow
question_answer 77) Optical isomerism is exhibited by (\[\text{oX}\,\text{=}\]oxalate anion; \[\text{en}\,\text{=}\]ethylenediamine).
A)
\[cis-{{[CrC{{l}_{2}}{{(ox)}_{2}}]}^{3-}}\]
done
clear
B)
\[{{[Co{{(en)}_{3}}]}^{3+}}\]
done
clear
C)
\[trans-{{[CrC{{l}_{2}}{{(ox)}_{2}}]}^{3-}}\]
done
clear
D)
\[{{[Co(ox){{(en)}_{2}}]}^{+}}\]
done
clear
View Answer play_arrow
question_answer 78) The increase in rate constant of a chemical reaction with increasing temperature is (are) due to the fact(s) that
A)
the number of collisions among the reactant molecules increases with increasing temperature
done
clear
B)
the activation energy of the reaction decreases with increasing temperature
done
clear
C)
the concentration of the reactant molecules increases with increasing temperature
done
clear
D)
the number of reactant molecules acquiring the activation energy increases with increasing temperature
done
clear
View Answer play_arrow
question_answer 79)
Within the list shown below, the correct pair of structures of alanine in pH ranges 2-4 and 9-11 is (i) \[{{H}_{3}}{{N}^{+}}-CH(C{{H}_{3}})C{{O}_{2}}H\] (ii) \[{{H}_{2}}N-CH(C{{H}_{3}})CO_{2}^{-}\] (iii) \[{{H}_{3}}{{N}^{+}}-CH(C{{H}_{3}})CO_{2}^{-}\] (iv) \[{{H}_{2}}N-CH(C{{H}_{3}})C{{O}_{2}}H\]
A)
I and II
done
clear
B)
I and III
done
clear
C)
II and III
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 80)
Identify the correct method for the synthesis of the compound shown below from the following alternatives.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 81) A set of genes will be in a complete linkage when the progeny phenotypes for parental (P) and recombinant (R) types are
A)
\[P=0%,R=100%\]
done
clear
B)
\[P=50%,\text{ }R=50%\]
done
clear
C)
\[P<50%,\text{ }R>50%\]
done
clear
D)
\[~P=100%,\text{ }R=0%\]
done
clear
View Answer play_arrow
question_answer 82) Which one of the following statements is wrong in relation to transgenic Bt cotton plant?
A)
Crop yield loss due to the attack by Bacillus thuringiensis bacterium is reduced
done
clear
B)
Crop yield loss due to the attack by lepidopteran insect pests is reduced
done
clear
C)
The use of chemical insecticides in the cotton field is minimised
done
clear
D)
Better quality cotton is produced
done
clear
View Answer play_arrow
question_answer 83) Which one of the following natural polymers is found both in insects and fungi?
A)
Pectin
done
clear
B)
Chitin
done
clear
C)
Cellulose
done
clear
D)
Suberin
done
clear
View Answer play_arrow
question_answer 84) Third stage larva of Wuchereria bancrofti carried by Culex mosquito is called
A)
cysticercus
done
clear
B)
merozoite
done
clear
C)
microfilariae
done
clear
D)
trophozoite
done
clear
View Answer play_arrow
question_answer 85) Persons suffering from sickle-cell anaemia normally do not suffer from
A)
cholera
done
clear
B)
malaria
done
clear
C)
high blood pressure
done
clear
D)
hepatitis
done
clear
View Answer play_arrow
question_answer 86) Two related but geographically isolated species sire known as
A)
sibling species
done
clear
B)
sympatric species
done
clear
C)
taxonomic species
done
clear
D)
allopatric species
done
clear
View Answer play_arrow
question_answer 87) Which hormone is responsible for reabsorption of water in kidney?
A)
ADH
done
clear
B)
STH
done
clear
C)
ACTH
done
clear
D)
GTH
done
clear
View Answer play_arrow
question_answer 88) Wildlife Protection Act India was implemented in the year
A)
1982
done
clear
B)
1988
done
clear
C)
1972
done
clear
D)
1970
done
clear
View Answer play_arrow
question_answer 89) Which one of the following is a causative agent of plague?
A)
Shigella flexneri
done
clear
B)
Bordetella pertussis
done
clear
C)
Staphylococcus aureus
done
clear
D)
Yersinia pestis
done
clear
View Answer play_arrow
question_answer 90) Which one of the following hormones is responsible for uterine contraction during parturition?
A)
Relaxin
done
clear
B)
Vasopressin
done
clear
C)
Oxytocin
done
clear
D)
Prolactin
done
clear
View Answer play_arrow
question_answer 91) Melatonin is produced from
A)
pineal gland
done
clear
B)
adrenal gland
done
clear
C)
parathyroid gland
done
clear
D)
ovary
done
clear
View Answer play_arrow
question_answer 92) Which one of the following is wrong for meiosis?
A)
It leads to formation of sister chromatids
done
clear
B)
It occurs in diploid cell
done
clear
C)
It occurs in haploid cell
done
clear
D)
lt occurs by splitting of centromeres and separation of sister chromatids
done
clear
View Answer play_arrow
question_answer 93) Which one of the following combination of all three fatty acids are essential for human beings?
A)
Oleic acid. linoleic acid and linolenic acid
done
clear
B)
Palmitic acid. linoleic acid and arachidonic acid
done
clear
C)
Oleic acid, linoleic acid and arachidonic acid
done
clear
D)
Linoleic acid, linolenic acid and arachidonic acid
done
clear
View Answer play_arrow
question_answer 94) Which one of the following information is essential to determine the genetic map distance between two genes located on the same chromosome?
A)
Length of the particular chromosome
done
clear
B)
Number of genes present in the particular chromosome
done
clear
C)
Number of nucleotides in the particular chromosome
done
clear
D)
Percentage of crossing over or recombinant frequency between the two genes
done
clear
View Answer play_arrow
question_answer 95) Phenomenon involving increase in concentration of non-degradable pollutants from lower to higher trophic levels is called
A)
biomagnification
done
clear
B)
bioaccumulation
done
clear
C)
biodegradation
done
clear
D)
bioinvasion
done
clear
View Answer play_arrow
question_answer 96) Which one of the following animals is uricotelic?
A)
Lizard
done
clear
B)
Camel
done
clear
C)
Toad
done
clear
D)
Rohu fish
done
clear
View Answer play_arrow
question_answer 97) Zymogenic cells of gastric gland secrete
A)
pepsinogen
done
clear
B)
trypsin
done
clear
C)
pepsin
done
clear
D)
chymotrypsin
done
clear
View Answer play_arrow
question_answer 98) During entry into the ovum, acrosome of sperm releases
A)
hyaluronidase
done
clear
B)
alkaline phosphatase
done
clear
C)
acid phosphatase
done
clear
D)
carbonic anhydrase
done
clear
View Answer play_arrow
question_answer 99) The epithelium found in the inner linings of stomach and intestine is
A)
columnar
done
clear
B)
squamous
done
clear
C)
stratified
done
clear
D)
pseudostratified
done
clear
View Answer play_arrow
question_answer 100) What is the stroke volume of an adult human heart?
A)
50 mL
done
clear
B)
70 mL
done
clear
C)
90 mL
done
clear
D)
100 Ml
done
clear
View Answer play_arrow
question_answer 101) Which one of the following cocci appears like grapes under microscope?
A)
Streptococci
done
clear
B)
Diplococci
done
clear
C)
Staphylococci
done
clear
D)
Pneumococci
done
clear
View Answer play_arrow
question_answer 102) Which one of the following components of urine in a healthy human does not differ much in concentration from that of blood plasma?
A)
\[N{{H}_{4}}^{+}\]
done
clear
B)
\[{{K}^{+}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[S{{O}_{4}}^{2-}\]
done
clear
View Answer play_arrow
question_answer 103) Antibodies produced by a group of identical B-cells against a single epitope of an antigen is called
A)
polyclonal antibodies
done
clear
B)
monoclonal antibodies
done
clear
C)
anti-hapten antibodies
done
clear
D)
somaclonal antibodies
done
clear
View Answer play_arrow
question_answer 104) During waste water treatment, trickling filter is used for
A)
primary treatment
done
clear
B)
secondary aerobic treatment
done
clear
C)
secondary anaerobic treatment
done
clear
D)
tertiary treatment
done
clear
View Answer play_arrow
question_answer 105) The apoplast is located
A)
outside the plasma membrane
done
clear
B)
in the entire cytosol
done
clear
C)
on both sides of plasma membrane
done
clear
D)
in the plastidial content
done
clear
View Answer play_arrow
question_answer 106) The aleurone synthesises and secretes digestive enzymes that hydrolyse nutrients stored in the endosperm, in the presence of
A)
auxin
done
clear
B)
gibberellin
done
clear
C)
cytokinin
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 107) ATP synthesis in cell requires
A)
\[{{H}^{+}}\] gradient across the membrane
done
clear
B)
\[{{K}^{+}}\] gradient across the membrane
done
clear
C)
\[P{{O}_{4}}^{3-}\] gradient across the membrane
done
clear
D)
\[C{{a}^{2+}}\]gradient across the membrane
done
clear
View Answer play_arrow
question_answer 108) Relationship between DO and BOD is that they
A)
are directly proportional
done
clear
B)
are inversely proportional
done
clear
C)
are not related
done
clear
D)
always remain equal to each other
done
clear
View Answer play_arrow
question_answer 109) What is the full form of MAB?
A)
Man and biosphere
done
clear
B)
Man and biosphere reserve
done
clear
C)
Man and biosphere reserve programme
done
clear
D)
Man and biosphere programme
done
clear
View Answer play_arrow
question_answer 110) The Red Data Book records
A)
species diversity of wetlands
done
clear
B)
list of water pollutants
done
clear
C)
list of threatened species
done
clear
D)
rate of population decline
done
clear
View Answer play_arrow
question_answer 111) Beta \[(\beta )\] diversity refers to diversity
A)
within a community
done
clear
B)
between communities
done
clear
C)
between two eco zones
done
clear
D)
within a population
done
clear
View Answer play_arrow
question_answer 112) Which one of the following acts solely as an inhibitory neurotransmitter?
A)
Norepinephrine
done
clear
B)
Gamma \[(\gamma )\] amino butyric acid
done
clear
C)
Acetylcholine
done
clear
D)
Dopamine
done
clear
View Answer play_arrow
question_answer 113) Which one of the following antibiotics kills bacterial cells by inhibiting the olymerisation of peptidoglycan?
A)
Aminoglycosides
done
clear
B)
Fluoroquinolones
done
clear
C)
Quinines
done
clear
D)
Penicillins
done
clear
View Answer play_arrow
question_answer 114) Indicate the correct sequence during spermatogenesis.
A)
Spermatozoa \[\to \] spermatogonia \[\to \]spermatid \[\to \]spermatocyte
done
clear
B)
Spermatogonia\[\to \]spermatocyte \[\to \] spermatid\[\to \] spermatozoa
done
clear
C)
Spermatid\[\to \]spermatocyte\[\to \] spermatozoa\[\to \]spermatogonia
done
clear
D)
Spermatocyte \[\to \] spermatozoa \[\to \]spermatid \[\to \] spermatogonia
done
clear
View Answer play_arrow
question_answer 115) Which one of the following is called intra-specific chemical messenger?
A)
Pheromones
done
clear
B)
Prostaglandins
done
clear
C)
Corticotrophin
done
clear
D)
Catecholamines
done
clear
View Answer play_arrow
question_answer 116) Egg in female gametophyte is accompanied by
A)
antipodal cells
done
clear
B)
synergids
done
clear
C)
definitive nucleus
done
clear
D)
tube nucleus
done
clear
View Answer play_arrow
question_answer 117) Malacophily is the pollination by
A)
insects
done
clear
B)
birds
done
clear
C)
snails
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 118) Grittiness of pear fruit is caused by
A)
sclereides
done
clear
B)
raphides
done
clear
C)
collenchyma
done
clear
D)
dead parenchyma cells
done
clear
View Answer play_arrow
question_answer 119) Which one of the following organisms is not used as a biocontrol agent?
A)
Bacillus sphaericus
done
clear
B)
Trichoderma viride
done
clear
C)
Bacillus thuringiensis
done
clear
D)
Bacillus subtilis
done
clear
View Answer play_arrow
question_answer 120) Which one of the following statements is relevant to sex linked characters?
A)
They always follow criss-cross inheritance
done
clear
B)
They do not follow criss-cross inheritance
done
clear
C)
They are mostly present on Y-chromosome
done
clear
D)
They are only present on X-chromosome
done
clear
View Answer play_arrow
question_answer 121) Which one of the following insecticides is of plant origin?
A)
Ecdysone
done
clear
B)
Rotenone
done
clear
C)
Parathion
done
clear
D)
Malathion
done
clear
View Answer play_arrow
question_answer 122) The resting state of reptiles in winter is
A)
hibernation
done
clear
B)
aestivation
done
clear
C)
diapause
done
clear
D)
moulting
done
clear
View Answer play_arrow
question_answer 123) Archaeopteryx is a connecting link between
A)
pisces and amphibians
done
clear
B)
amphibians and reptiles
done
clear
C)
reptiles and birds
done
clear
D)
birds and mammals
done
clear
View Answer play_arrow
question_answer 124) Which one of the following secretes glucagon?
A)
Beta \[(\beta )\] cells of islets of Langerhans
done
clear
B)
Alpha \[(\alpha )\] cells of islets of Langerhans
done
clear
C)
Acidophilic cells of adenohypophysis
done
clear
D)
Basophilic cells of adenohypophysis
done
clear
View Answer play_arrow
question_answer 125) Osteoid refers to
A)
the smallest bone of the body
done
clear
B)
young hyaline matrix of true bone in which calcium salts are deposited
done
clear
C)
membranous ossification of cranium
done
clear
D)
the largest bone of the body
done
clear
View Answer play_arrow
question_answer 126) The bundle of axons in the central nervous system is known as
A)
nerve
done
clear
B)
ganglion
done
clear
C)
tract
done
clear
D)
neuron
done
clear
View Answer play_arrow
question_answer 127) Which one of the following enzymes is responsible for the conversion of norepinephrine to epinephrine?
A)
Catecholamine-O-methyltransferase
done
clear
B)
Phenylalanine-N-methyltransferase
done
clear
C)
DOPA decarboxylase
done
clear
D)
Monoamine oxidase
done
clear
View Answer play_arrow
question_answer 128) Passage cells help in
A)
transport of water towards pericycle
done
clear
B)
transport of water towards epiblema
done
clear
C)
absorption of water from soil
done
clear
D)
passage of\[\text{C}{{\text{O}}_{\text{2}}}\] towards stomata
done
clear
View Answer play_arrow
question_answer 129) Medullary rays are tissues made up of
A)
phloem parenchyma
done
clear
B)
xylem parenchyma
done
clear
C)
sieve tube
done
clear
D)
sclerenchyma
done
clear
View Answer play_arrow
question_answer 130) An allosteric inhibitor of the enzyme acts by binding to the
A)
substrate
done
clear
B)
product
done
clear
C)
catalytic site of the enzyme
done
clear
D)
non-catalytic site of the enzyme
done
clear
View Answer play_arrow
question_answer 131) Which one of the followings is an in situ method of biodiversity conservation?
A)
National park
done
clear
B)
Botanical garden
done
clear
C)
Zoological garden
done
clear
D)
Scientific laboratory
done
clear
View Answer play_arrow
question_answer 132) Nucleosome contains
A)
Only histone protein
done
clear
B)
Both DNA and histone protein
done
clear
C)
Only DNA
done
clear
D)
Both DNA and RNA
done
clear
View Answer play_arrow
question_answer 133) Which one of the followingmatchmg pairs is incorrect?
A)
Mollusca-Pseudocoet
done
clear
B)
Cnidaria-Nematocyst
done
clear
C)
Annelida-Chloragogen cells
done
clear
D)
Echinodermata-Water vascular system
done
clear
View Answer play_arrow
question_answer 134) Which one of the following matching pairs is incort-ect?
A)
Shellfish - Pisces
done
clear
B)
Silver fish - Arthropoda
done
clear
C)
Cuttle fish - Mollusca
done
clear
D)
Star fish - Echinodermata
done
clear
View Answer play_arrow
question_answer 135) All of the following symptoms are found in jaundice except
A)
disorders of hepato-biliary system
done
clear
B)
abnormal secretion of pancreatic and gastric juices
done
clear
C)
bile duct obstruction
done
clear
D)
anaemia
done
clear
View Answer play_arrow
question_answer 136) The hormone that stimulates the release of pancreatic juice is
A)
secretin
done
clear
B)
glucagon
done
clear
C)
inhibin
done
clear
D)
insulin
done
clear
View Answer play_arrow
question_answer 137) Which one of the following combinations acts as a usual antigen binding site of an antibody?
A)
Variable regions of a light and another heavy chain
done
clear
B)
Variable regions of two. light chains
done
clear
C)
Variable regions of two heavy chains .
done
clear
D)
Variable -region -of a heavy chain and constant region of a light chain
done
clear
View Answer play_arrow
question_answer 138) Nitrogenase enzyme is a
A)
magnesium-iron protein
done
clear
B)
molybdenum-iron protein
done
clear
C)
iron-copper protein
done
clear
D)
nickel-iron protein
done
clear
View Answer play_arrow
question_answer 139) Necrosis (die-back) of-the tip of young leaves is caused due to the deficiency of
A)
iron
done
clear
B)
manganese
done
clear
C)
zinc
done
clear
D)
copper
done
clear
View Answer play_arrow
question_answer 140) Guttation is a process of loss of water in
A)
liquid form containing dissolved minerals
done
clear
B)
liquid form without dissolved minerals
done
clear
C)
vapour form with minerals
done
clear
D)
vapour form without minerals
done
clear
View Answer play_arrow
question_answer 141) What will be the percentage of guanine in a DNA molecule having 20% adenine?
A)
20%
done
clear
B)
30%
done
clear
C)
40%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 142) Which one of the following group of animals is homeothermic?
A)
Reptiles
done
clear
B)
Amphibians
done
clear
C)
Birds
done
clear
D)
Fishes
done
clear
View Answer play_arrow
question_answer 143) Neoteny refers to
A)
development of gonads
done
clear
B)
moulting
done
clear
C)
metamorphosis
done
clear
D)
retention of larval traits in the adult body
done
clear
View Answer play_arrow
question_answer 144) The overlapping zone in between two ecosystems is known as
A)
ecozone
done
clear
B)
biotope
done
clear
C)
ecotone
done
clear
D)
buffer zone
done
clear
View Answer play_arrow
question_answer 145) The animal species controlling the ecosystem functioning is known as
A)
edge species
done
clear
B)
pioneer species
done
clear
C)
keystone species
done
clear
D)
umbrella species
done
clear
View Answer play_arrow
question_answer 146) Central dogma in molecular biology is
A)
RNA \[\to \] DNA \[\to \] Protein
done
clear
B)
DNA \[\to \] RNA \[\to \]Protein
done
clear
C)
RNA \[\to \]Protein \[\to \] DNA
done
clear
D)
DNA \[\to \] Protein\[\to \]RNA
done
clear
View Answer play_arrow
question_answer 147) Which one of the followings is the functional unit of hearing?
A)
Utricle
done
clear
B)
Organ of Zuckerkandl
done
clear
C)
Organ of Corti
done
clear
D)
Vestibular apparatus
done
clear
View Answer play_arrow
question_answer 148) Which one of the followings is not a refractive medium of the eye?
A)
Lens
done
clear
B)
Vitreous humour
done
clear
C)
Aqueous humour
done
clear
D)
Pupil
done
clear
View Answer play_arrow
question_answer 149) The heart is covered by
A)
epicardium
done
clear
B)
pericardium
done
clear
C)
supracardium
done
clear
D)
endocardium
done
clear
View Answer play_arrow
question_answer 150) Vernalisation promotes flowering by
A)
low temperature
done
clear
B)
high temperature
done
clear
C)
prolonged photoperiod
done
clear
D)
short photoperiod
done
clear
View Answer play_arrow
question_answer 151) \[{{\text{C}}_{\text{4}}}\]pathway is advantageous over \[{{\text{C}}_{3}}\]pathway in plants as it
A)
occurs in relatively low \[\text{C}{{\text{O}}_{\text{2}}}\]concentration
done
clear
B)
uses more amount of water
done
clear
C)
occurs in relatively low \[{{\text{O}}_{2}}\] concentration
done
clear
D)
is less efficient in energy utilisation
done
clear
View Answer play_arrow
question_answer 152) TCA cycle enzymes are located in
A)
cristae .
done
clear
B)
outer membrane
done
clear
C)
mitochondrial matrix
done
clear
D)
mitochondrial intermembrane space
done
clear
View Answer play_arrow
question_answer 153) Which one of the following statements is incorrect?
A)
Insects have one pair of antenna
done
clear
B)
Millipeds possess two pairs of appendages in each segment of the body
done
clear
C)
Prawns have two pairs of antenna
done
clear
D)
Animals belonging to th8 phylum-Porifera have nematocyst
done
clear
View Answer play_arrow
question_answer 154) Which one of the followings is not a characteristic feature of mammals?
A)
Diphyodont tooth
done
clear
B)
Ten pairs of cranial nerves
done
clear
C)
Seven cervical vertebrae
done
clear
D)
Left aortic arch in the circulatory system
done
clear
View Answer play_arrow
question_answer 155) Which one of the following combinations is incorrect?
A)
Rio convention - Air pollution
done
clear
B)
Kyoto protocol - Climate change
done
clear
C)
Montreal protocol - Ozone depletion
done
clear
D)
Ramsar - Wetland convention conservation
done
clear
View Answer play_arrow
question_answer 156) The eukaryotic cells have all of the followings except
A)
peptidoglycan in the cell wall
done
clear
B)
the 80 S ribosome
done
clear
C)
nuclear membrane
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 157) Which of the followings does not occur in the interphase of eukaryotic cell division?
A)
Increase of ATP synthesis
done
clear
B)
Increase of DNA synthesis
done
clear
C)
Jncrease of RNA synthesis
done
clear
D)
Reduction in cell size
done
clear
View Answer play_arrow
question_answer 158) Lactose (Lac) operon is regulated by
A)
lac repfessor only
done
clear
B)
lac represser and CAP-cGM P complex
done
clear
C)
lac,repressor and CAP-cAMP complex
done
clear
D)
CAP-cAMP and CAP-cGMP complex
done
clear
View Answer play_arrow
question_answer 159) Elongation of internode is caused by
A)
ethylene
done
clear
B)
gibberellin
done
clear
C)
abscisic acid
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 160) Endosperm nucleus is
A)
n
done
clear
B)
2n
done
clear
C)
3n
done
clear
D)
4n
done
clear
View Answer play_arrow
question_answer 161) Banana is an example of
A)
parthenocarpy
done
clear
B)
apomixis
done
clear
C)
parthenogenesis
done
clear
D)
polyembryony
done
clear
View Answer play_arrow
question_answer 162) Stock and scion are used in
A)
cutting
done
clear
B)
grafting
done
clear
C)
layering
done
clear
D)
micropropagatfbn
done
clear
View Answer play_arrow
question_answer 163) Which one of the following is correct for blooming of short dayplants?
A)
The long dark period is not critical
done
clear
B)
It is affected by intengaption of long dark period by brief exposure of light
done
clear
C)
It is not affected by interruption of long dark period by brief exposure of light
done
clear
D)
It is affected if the continuous light period is interrupted
done
clear
View Answer play_arrow
question_answer 164) A dicotyledonous plant forms crown gall when
A)
Agrobacterium tumefaciens comes in contact with the plant
done
clear
B)
Agrobacterium rhizogenes come$Jn contact with the plant
done
clear
C)
a specific part of DNA from the Ti-plasmid gets fntegrated with the plant chromosome
done
clear
D)
a specific part of DNA from the Ri-plasmid gets integrated with the plant chromosome
done
clear
View Answer play_arrow
question_answer 165) Gene therapy has been successful in curing genetic diseases in laboratory animals through
A)
exposure to X-ray to rectify the defective gene
done
clear
B)
replacing the defective gene with a functional gene
done
clear
C)
oral delivery of genes
done
clear
D)
use of therapeutic medicines to rectify the defective gene
done
clear
View Answer play_arrow
question_answer 166) The enzyme peptidyl transferase of prokaryotes resides in
A)
50 S ribosome
done
clear
B)
30 S ribosome
done
clear
C)
40 S ribosome
done
clear
D)
60 S ribosome
done
clear
View Answer play_arrow
question_answer 167) Which one of the followings is correct for the trans membrane proteins in lipid bilayer?
A)
They are absent in animal cells
done
clear
B)
They act as channel proteins
done
clear
C)
They are absent in plant cells
done
clear
D)
They are only externally located
done
clear
View Answer play_arrow
question_answer 168) Engulfing of solid materials by cells is called?
A)
pinocytosis
done
clear
B)
phagocytosis
done
clear
C)
active transport
done
clear
D)
autolysis
done
clear
View Answer play_arrow
question_answer 169) The £RNA anticodon 3-UAC-5will pair with the mRNA codon
A)
5-AUU-3
done
clear
B)
5-UAC-3
done
clear
C)
5-AUG-3
done
clear
D)
3-GUA-5
done
clear
View Answer play_arrow
question_answer 170) Peroxisomes have
A)
ribosome
done
clear
B)
DNA
done
clear
C)
catalase enzyme
done
clear
D)
centrosome
done
clear
View Answer play_arrow
question_answer 171) Two membrane envelops is found in
A)
mitochondria, Golgi apparatus and chloroplast
done
clear
B)
mitochondria, nucleus and chloroplast
done
clear
C)
nucleus, Golgi apparatus and endoplasmic reticulum
done
clear
D)
nucleus, ribosome andchloroplast
done
clear
View Answer play_arrow
question_answer 172)
Match the items in column I with those in column II and choose the correct answer.
Column I
Column II
A.
Thiobacillus
1.
Nitrogen-fixation
B.
Nitrosomonas
2.
Ammonification
C.
Azotobacter
3.
Nitrification
D.
Pseudomonas
4.
Denitrification
A)
A-4B-3C-1D-2
done
clear
B)
A-3B-4C-1D-2
done
clear
C)
A-4B-2C-1D-3
done
clear
D)
A-2B-1C-3D-4
done
clear
View Answer play_arrow
question_answer 173)
Match the item in column I with those in column II and the choose the correct answer. Column I Column II A. Mitosis 1. Occurs in diploid cells only B. Meiosis 2. Occurs in both haploid and diploid cells 3. Daughter and parent cells have same chromosome numbers 4. Synapsis of homologous chromosomes
A)
A-1 B-2
done
clear
B)
A-2 B-3
done
clear
C)
A-3 B-4
done
clear
D)
A-4 B-1
done
clear
View Answer play_arrow
question_answer 174) A male rabbit of genotype AABBDDEE is crossed with a female rabbit of genotype aabbddee to produce \[{{F}_{1}}\]hybrid offspring. How many genetically different gametes can be produced by this \[{{F}_{1}}\]hybrid?
A)
4
done
clear
B)
8
done
clear
C)
16
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 175)
Match the following I with column II. Column I Column II A. Pollen grains 1. Photochemical smog B. PAN 2. Particulate pollution c. \[C{{O}_{2}}\] 3. Global warming D. Cadmium 4. Itai-itai disease
A)
A-2 B-1 C-3 D-4
done
clear
B)
A-4 B-2 C-1 D-3
done
clear
C)
A-1 B-2 C-3 D-4
done
clear
D)
A-3 B-1 C-2 D-4
done
clear
View Answer play_arrow
question_answer 176)
Select correct combination of statements for lymph. (I) It helps to maintain fluid balance of the body. (II) It is contained in lymphatic vessels and lymphatic organs in mammals. (III) It is derived from tissue fluid. (IV) It contains less antibodies than plasma. (V) Flows in both directions. (VI) It helps to conserve proteins and remove bacteria.
A)
I, II, III, V
done
clear
B)
II, III, IV, VI
done
clear
C)
l,IV,V,VI
done
clear
D)
III, IV. V, VI
done
clear
View Answer play_arrow
question_answer 177)
Match the following column I with column II.
Column I
Column II
A.
Cytology
1.
Study of fossils
B.
Entomology
2.
Study of cells
C.
Palaeontology
3.
Study of birds
D.
Ornithology
4.
Study of insects
A)
A-2 B-3 C-4 D-1
done
clear
B)
A-2 B-4 C-1 D-3
done
clear
C)
A-1 B-2 C-4 D-3
done
clear
D)
A-3 B-2 C-1 D-4
done
clear
View Answer play_arrow
question_answer 178) Genes for maternal inheritance are located-in
A)
Golgi bodies
done
clear
B)
mitochondria
done
clear
C)
lysosome
done
clear
D)
nucleolus
done
clear
View Answer play_arrow
question_answer 179)
Match the following column I with column II.
Column I
Column II
A.
Producer
1.
Herbivores
B.
Primary consumer
2.
Green plants
C.
Secondary consumer
3.
Saprotrophs
D.
Decomposer
4.
Carnivores
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-2 B-1 C-4 D-3
done
clear
C)
A-2 B-4 C-3 D-1
done
clear
D)
A-3 B-2 C-1 D-4
done
clear
View Answer play_arrow
question_answer 180)
Match the following column I with column II. Column I Column II A. Vitamin-\[{{B}_{1}}\] 1. Accumulation of fat B. Gastric juice 2. Loss of fat C. Starvation 3. Pepsin D. Obesity 4. Beri-beri
A)
A-3 B-4 C-2 D-1
done
clear
B)
A-3 B-4 C-1 D-2
done
clear
C)
A-4 B-3 C-2 D-1
done
clear
D)
A-4 B-2 C-3 D-1
done
clear
View Answer play_arrow
question_answer 181)
Select the correct combination of statements for DNA fingerprinting. (i) It is ELISA based technique. (ii) It is PCR based technique. (iii) It is used by forensic scientists. (IV) It is based on the fingerprint of the individual. (V) It is a test for paternity.
A)
I, II and III
done
clear
B)
II, III and IV
done
clear
C)
I, IV and V
done
clear
D)
I, III and IV
done
clear
View Answer play_arrow
question_answer 182)
Match the items in column I with those in column II and choose the correct answer. Column I Column II A. Klinefelter syndrome 1. Mutation in autosomal gene B. Thalassaemia 2. Mutation in sex chromosome-linked gene C. Down syndrome 3. Trisomy of autosome D. Colour blindness 4. Trisomy of sex chromosome
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-2 B-3 C-4 D-1
done
clear
C)
A-3 B-4 C-1 D-2
done
clear
D)
A-4 B-1 C-3 D-2
done
clear
View Answer play_arrow
question_answer 183) An area is declared as Hot Spot when
A)
it has 1500 or more endemic species and 75% of its original habitat is lost
done
clear
B)
it has 1500 or more vertebrate species and 75% of its original habitat is lost
done
clear
C)
it has more than 2000 species of plants
done
clear
D)
most of the species inhabiting the area is facing the risk of extinction
done
clear
View Answer play_arrow
question_answer 184)
Select the correct combination of statements regarding Myasthenia gra vis. (i) It is an autoimmune disorder. (ii) It causes insufficient acetylcholine binding that affects muscular contraction. (iii) Antibodies are developed against acetylcholine. (IV) Antibodies are developed Against acetylcholine receptors. (V) Antibodies are developed against acetylcholine esterase. (VI) It causes drooping of eyelids.
A)
I, III, IV, VI
done
clear
B)
I, III, IV, VI
done
clear
C)
I, II, IV, VI
done
clear
D)
II, III, IV, V
done
clear
View Answer play_arrow
question_answer 185) Each 100 mL of human arterial blood carries T mL of \[{{\text{O}}_{\text{2}}}\] and Q mL of \[\text{C}{{\text{O}}_{\text{2}}},\]whereas each 100 mL of venous blood carries R mL of \[{{\text{O}}_{\text{2}}}\] and S mL of \[\text{C}{{\text{O}}_{\text{2}}}.\]Choose the correct values ofP,Q, R and S.
A)
\[P=48mL,Q=19-20mL,R=52mL,S=14-15mL\]
done
clear
B)
\[P=19-20mL,Q=48mL,R=14-15mL,S=52mL\]
done
clear
C)
\[P=14-15mL,Q=52mL,R=19-20mL,S=48mL\]
done
clear
D)
\[P=52mL,Q=14-15mL,R=48mL,S=19-20mL\]
done
clear
View Answer play_arrow
question_answer 186) The usual cause (s) of peptic ulceration is/are
A)
lower rate of secretion of gastric juice
done
clear
B)
higher rate of secretion of gastric and duodenal juices
done
clear
C)
improper neutralisation of gastric juice by duodenal juices
done
clear
D)
imbalance between the rate of secretion of gastric juice and the degree of protection offered by gastro-duoctenal mucosa
done
clear
View Answer play_arrow
question_answer 187) Which of the following statements, is/are correct regarding the effects of pH on enzyme catalysed, reactions?
A)
Direction of the reaction is influenced by \[[{{H}^{+}}].\]
done
clear
B)
lonisation state of dissociating groups on the enzyme is modified.
done
clear
C)
lonisation state of the substrate is modified.
done
clear
D)
Protein is not denatured with the change in pH.
done
clear
View Answer play_arrow
question_answer 188) Which of the following statements is/are correct for transduction?
A)
It is observed in Gram positive and Gram negative bacteria.
done
clear
B)
Bacteria should be in state of competence.
done
clear
C)
Transfer of DNA by a bacteriophagp takes place.
done
clear
D)
Packaging of both host and phage DNA takes place.
done
clear
View Answer play_arrow
question_answer 189) Select the correct statement (s) pertaining to Chipko movement.
A)
It was led by Sunderial Bahuguna.
done
clear
B)
It was a tree hugging movement.
done
clear
C)
It commenced in the Tehri-Garhwal district.
done
clear
D)
lt received global attention on environmentalprotection.
done
clear
View Answer play_arrow
question_answer 190) Select the correct combination (s) from the following.
A)
Gir - Asiatic Lion
done
clear
B)
Sunderbans - Rhinoceros
done
clear
C)
Periyar - Indian Elephant
done
clear
D)
Corbet National - Red Panda Park
done
clear
View Answer play_arrow
question_answer 191) Select the non-degradable pollutant (s) from the followings.
A)
Plastic
done
clear
B)
Organochlorine pesticides
done
clear
C)
Heavymetals
done
clear
D)
Domestic sewage
done
clear
View Answer play_arrow
question_answer 192) Select the correct combination (s) from the followings.
A)
Encephalitis - Viral disease
done
clear
B)
Kala-azar - Phlebotomus
done
clear
C)
Rhabditiform larvae -Ascaris
done
clear
D)
Entamoeba - Sporogony
done
clear
View Answer play_arrow
question_answer 193) Intrinsic and extrinsic pathways of blood clotting are interlinked at the activation step of which of the following factors?
A)
Factor IX
done
clear
B)
Factor IV
done
clear
C)
Factor X
done
clear
D)
Factor Xllla
done
clear
View Answer play_arrow
question_answer 194) Which of the following pairs of cranial nerves is/are of mixed category?
A)
Glossppharyngeal and hypoglossal
done
clear
B)
Trigeminal and abducens
done
clear
C)
Trigeminal and facial
done
clear
D)
Giossopharyngeal and vagus
done
clear
View Answer play_arrow
question_answer 195) Opening and closing of stomata is controlled by
A)
abscisic acid
done
clear
B)
\[\text{C}{{\text{O}}_{\text{2}}}\]concentration
done
clear
C)
concentration
done
clear
D)
light intensity
done
clear
View Answer play_arrow
question_answer 196) Which of these gases was/were present in prebiotic atmosphere?
A)
Ammonia
done
clear
B)
Methane
done
clear
C)
Oxygen
done
clear
D)
Hydrogen
done
clear
View Answer play_arrow
question_answer 197) Which of these components is/are not present in Gram-negative bacteria?
A)
Teichoic acid
done
clear
B)
Pseudomurein
done
clear
C)
Lipopolysaccharide
done
clear
D)
Mycolic acid
done
clear
View Answer play_arrow
question_answer 198) Which of the following features is/are correct for heterochromatin of eukaryotic nucleus?
A)
It is highly expanded in interphase
done
clear
B)
It stains densely with basic dyes
done
clear
C)
It is highly condensed in interphase
done
clear
D)
It stains densely with acidic dyes
done
clear
View Answer play_arrow
question_answer 199) Which of the followings is/are correct for the inheritance of genes involved in human ABCY blood grouping?
A)
It is inherited by complete dominant allele
done
clear
B)
It is inherited by complete recessive allele
done
clear
C)
It is inherited by codominant allele
done
clear
D)
It is inherited by single gene with more than two alleles
done
clear
View Answer play_arrow
question_answer 200) Antelope cervicapra is
A)
a mammal
done
clear
B)
commonly known as black back
done
clear
C)
an animal under data deficient category of wildlife
done
clear
D)
a threatened Indian wildlife
done
clear
View Answer play_arrow
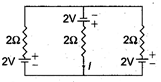
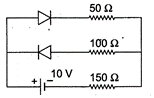
 Their total angular momentum about the point \[O\] is
Their total angular momentum about the point \[O\] is
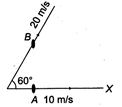
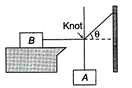
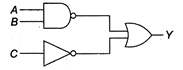

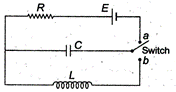
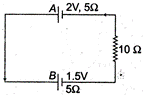
 The major product of the above reaction is
The major product of the above reaction is
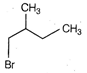
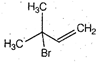
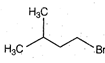
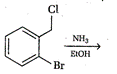 The product of the above reaction is
The product of the above reaction is
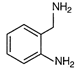
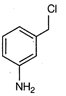
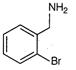
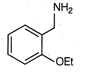
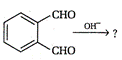 The product of the above reaction is
The product of the above reaction is