question_answer 1) The angle subtended by the vector \[A=4\hat{i}+3\hat{j}+12\hat{k}\]with the \[x-\]axis is
A)
\[{{\sin }^{-1}}\left( \frac{3}{13} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{4}{13} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{4}{13} \right)\]
done
clear
D)
\[{{\cos }^{-1}}\left( \frac{3}{13} \right)\]
done
clear
View Answer play_arrow
question_answer 2) The velocity of a particle v at an instant t is given by \[v=at+b{{t}^{2}}\]the dimension of b is
A)
[L]
done
clear
B)
\[[L{{T}^{-1}}]\]
done
clear
C)
\[[L{{T}^{-2}}]\]
done
clear
D)
\[[L{{T}^{-3}}]\]
done
clear
View Answer play_arrow
question_answer 3) The distance travelled by an object along a straight line in time t is given by\[s=3-4t+5{{t}^{2}},\] the initial velocity of the object is
A)
3 unit
done
clear
B)
- 3 unit
done
clear
C)
4 unit
done
clear
D)
- 4 unit
done
clear
View Answer play_arrow
question_answer 4) A pellet of mass 1 g is moving with an angular velocity of 1 rad/s along a circle of radius 1 m the centrifugal force is
A)
0.1 dyne
done
clear
B)
1 dyne
done
clear
C)
10 dyne
done
clear
D)
100 dyne
done
clear
View Answer play_arrow
question_answer 5) Two point objects of masses 1.5 g and 2.5 g respectively are at a distance of 16 cm apart, the centre of gravity is at a distance \[x\]from the object of mass 1.5 g where \[x\] is
A)
10 cm
done
clear
B)
6 cm
done
clear
C)
13 cm
done
clear
D)
3 cm
done
clear
View Answer play_arrow
question_answer 6) If the earth shrinks such that its mass does not change but radius decreases to one quarter of its original value then one complete day will take
A)
96 h
done
clear
B)
48 h
done
clear
C)
6 h
done
clear
D)
1.5 h
done
clear
View Answer play_arrow
question_answer 7) A shell of mass 10 kg is moving with a velocity of \[10\,\text{m}{{\text{s}}^{-1}}\] when it blasts and forms two parts of mass 9 kg and 1 kg respectively. If the 1st mass is stationary, the velocity of the \[\text{2}\,\text{nd}\] is
A)
1 m/s
done
clear
B)
10 m/s
done
clear
C)
100 m/s
done
clear
D)
1000 m/s
done
clear
View Answer play_arrow
question_answer 8) Force required to move a mass of 1 kg at rest on a horizontal rough plane (\[\mu =0.1\] and\[g=9.8\,m{{s}^{-2}}\]) is
A)
0.98 N
done
clear
B)
0.49 N
done
clear
C)
9.8 N
done
clear
D)
4.9 N
done
clear
View Answer play_arrow
question_answer 9) A rocket of mass 100 kg bums 0.1 kg of fuel per sec. If velocity of exhaust gas is 1 km/s, then it lifts with an acceleration of
A)
\[1000\,\text{m}{{\text{s}}^{-2}}\]
done
clear
B)
\[100\,\text{m}{{\text{s}}^{-2}}\]
done
clear
C)
\[10\,\text{m}{{\text{s}}^{-2}}\]
done
clear
D)
\[\text{1}\,\text{m}{{\text{s}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 10) The weight of a body on surface of earth is 12.6 N. When it is raised to a height half the radius of earth its weight will be
A)
2.8 N
done
clear
B)
5.6 N
done
clear
C)
12.5 N
done
clear
D)
25.2 N
done
clear
View Answer play_arrow
question_answer 11) A beaker of radius 15 cm is filled with a liquid of surface tension 0.75 N/m. Force across an imaginary diameter on the surface of the liquid is
A)
\[0.075\,N\]
done
clear
B)
\[1.5\times {{10}^{-2}}N\]
done
clear
C)
\[0.225\,N\]
done
clear
D)
\[2.25\times {{10}^{-2}}\,N\]
done
clear
View Answer play_arrow
question_answer 12) Two springs are joined and attached to a mass of 16 kg. The system is then suspended vertically from a rigid support. The spring constant of the two springs are \[{{k}_{1}}\]and \[{{k}_{2}}\] respectively. The two springs are \[{{k}_{1}}\]and \[{{k}_{2}}\]respectively. The period of vertical oscillations of the system will be
A)
\[\frac{1}{8\pi }\sqrt{{{k}_{1}}+{{k}_{2}}}\]
done
clear
B)
\[8\pi \sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{{{k}_{1}}{{k}_{2}}}}\]
done
clear
C)
\[\frac{\pi }{2}\sqrt{{{k}_{1}}-{{k}_{2}}}\]
done
clear
D)
\[\frac{\pi }{2}\sqrt{\frac{{{k}_{1}}}{{{k}_{2}}}}\]
done
clear
View Answer play_arrow
question_answer 13) The equation of a progressive wave can be given by\[y=15\]\[\sin (660\pi t-0.02\pi x)\,cm.\] The frequency of the wave is
A)
330 Hz
done
clear
B)
342 Hz
done
clear
C)
365 Hz
done
clear
D)
660 Hz
done
clear
View Answer play_arrow
question_answer 14) A hollow cylinder with both sides open generates a frequency \[f\] in air. When the cylinder vertically immersed into water by half its length the frequency will be
A)
\[f\]
done
clear
B)
\[2f\]
done
clear
C)
\[f/2\]
done
clear
D)
\[f/4\]
done
clear
View Answer play_arrow
question_answer 15) Two stretched strings have lengths; and 21 while tension are T and 4T respectively. If they are made of same material the ratio of their frequency is
A)
2:1
done
clear
B)
1:2
done
clear
C)
1 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 16) When sound is produced in an aeroplane moving with a velocity of 200 m/s horizontal its echo is heard after \[10\sqrt{5}\,s.\] If velocity of sound in air is the elevation of aircraft is
A)
250 m
done
clear
B)
\[250\sqrt{5}\,m\]
done
clear
C)
\[12.50\,m\]
done
clear
D)
2500 m
done
clear
View Answer play_arrow
question_answer 17) Two tuning forks of frequency \[{{n}_{1}}\]and \[{{n}_{2}}\]produces n beats per second. If \[{{n}_{2}}\]and n are known, \[{{n}_{1}}\]may be given by
A)
\[\frac{{{n}_{2}}}{n}+{{n}_{2}}\]
done
clear
B)
\[{{n}_{2}}n\]
done
clear
C)
\[{{n}_{2}}\pm n\]
done
clear
D)
\[\frac{{{n}_{2}}}{n}-{{n}_{2}}\]
done
clear
View Answer play_arrow
question_answer 18) A car moving with a velocity of 36 km/h crosses a siren of frequency 500 Hz. The apparent frequency of siren after passing it will be
A)
520 Hz
done
clear
B)
485 Hz
done
clear
C)
540 Hz
done
clear
D)
460 Hz
done
clear
View Answer play_arrow
question_answer 19) Six molecules speeds 2 unit, 5 unit, 3 unit, 6 units, 3 unit and 5 unit respectively. The rms speed is
A)
4 unit
done
clear
B)
1.7 unit
done
clear
C)
4.2 unit
done
clear
D)
5 unit
done
clear
View Answer play_arrow
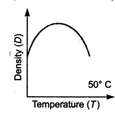
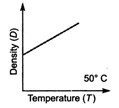
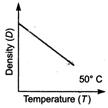
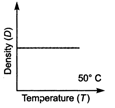
question_answer 20) Which one of the figure gives the temperature dependence of density of water correctly?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 21) A bullet emerge from a barrel of length 1.2 m with a speed of \[640\,\text{m}{{\text{s}}^{-1}}.\]Assuming constant acceleration, the approximate time that it spends in the barrel after the gun is fired is
A)
4 ms
done
clear
B)
40 ms
done
clear
C)
400 us
done
clear
D)
1s
done
clear
View Answer play_arrow
question_answer 22) A body of mass 3 kg acted upon by a constant force is displaced by S metre, given by relation \[S=\frac{1}{3}{{t}^{2}},\]where t is in second. Work done by the force in 2 is
A)
\[\frac{8}{3}J\]
done
clear
B)
\[\frac{19}{5}J\]
done
clear
C)
\[\frac{5}{19}J\]
done
clear
D)
\[\frac{3}{8}\,J\]
done
clear
View Answer play_arrow
question_answer 23) The ionisation potential of hydrogen atom is -13.6 eV. An electron in the ground state of a hydrogen atoms absorbs a photon of energy 12.75 eV. How many different spectral line can one expect when the electron make a downward transition?
A)
1
done
clear
B)
4
done
clear
C)
2
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 24) A piece of wood is floating in water. When the temperature of water rises, the apparent weight of the wood will
A)
increase
done
clear
B)
decrease
done
clear
C)
may increase or decrease
done
clear
D)
remain same
done
clear
View Answer play_arrow
question_answer 25) An experiment takes 10 min to raise v temperature of water from \[\text{0}{{\,}^{\text{o}}}\text{C}\]and \[\text{100}{{\,}^{\text{o}}}\text{C}\]and another 55 min to convert it totally into steam by a stabilized heater. The latent heat of vaporization comes out to be
A)
530 cal/g
done
clear
B)
540 cal/g
done
clear
C)
550 cal/g
done
clear
D)
560 cal/g
done
clear
View Answer play_arrow
question_answer 26) Which of the following substance has the highest elasticity?
A)
Steel
done
clear
B)
Copper
done
clear
C)
Rubber
done
clear
D)
Sponge
done
clear
View Answer play_arrow
question_answer 27) A wire is stretched under a force. If the wire suddenly snaps the temperature of the wire.
A)
remains the same
done
clear
B)
decrease
done
clear
C)
increase
done
clear
D)
first decrease then increase
done
clear
View Answer play_arrow
question_answer 28) When the room temperature becomes equal to the dew point, the relative humidity of the room is
A)
100%
done
clear
B)
zero %
done
clear
C)
70%
done
clear
D)
85 %
done
clear
View Answer play_arrow
question_answer 29) At what temperature will the rms speed of air molecules be double that NTP?
A)
\[\text{519}{{\,}^{\text{o}}}\text{C}\]
done
clear
B)
\[\text{619}{{\,}^{\text{o}}}\text{C}\]
done
clear
C)
\[\text{719}{{\,}^{\text{o}}}\text{C}\]
done
clear
D)
\[\text{819}{{\,}^{\text{o}}}\text{C}\]
done
clear
View Answer play_arrow
question_answer 30) The refractive indices of glass and quartz w. r. t. air are 3/2 and 12/5 respectively. The refractive index of quartz w. r. t. glass is
A)
8/5
done
clear
B)
5/8
done
clear
C)
5/18
done
clear
D)
18/5
done
clear
View Answer play_arrow
question_answer 31) The radius of curvature of concave mirror is 24 cm and the image is magnified by 1.5 times. The object distance is
A)
20 cm
done
clear
B)
8 cm
done
clear
C)
16 cm
done
clear
D)
24 cm
done
clear
View Answer play_arrow
question_answer 32) A point source of light is kept at a depth of h in water of refractive index 4/3. The radius of the circle at the surface of water through which light emits is
A)
\[\frac{3}{\sqrt{7}}h\]
done
clear
B)
\[\frac{\sqrt{7}}{3}h\]
done
clear
C)
\[\frac{\sqrt{3}}{7}h\]
done
clear
D)
\[\frac{7}{\sqrt{3}}h\]
done
clear
View Answer play_arrow
question_answer 33) 4 point charges each +q is placed on the circumference of a circle of diameter 2d in, such a way that they form a square. The potential at the centre is
A)
0
done
clear
B)
\[\frac{4q}{d}\]
done
clear
C)
\[\frac{4d}{q}\]
done
clear
D)
\[\frac{q}{4d}\]
done
clear
View Answer play_arrow
question_answer 34) 64 identical spheres of charge q and capacitance C each are combined to form a large sphere is The charge and capacitance of the large sphere is
A)
\[64q,C\]
done
clear
B)
\[16q,4C\]
done
clear
C)
\[64q,4C\]
done
clear
D)
\[16q,64C\]
done
clear
View Answer play_arrow
question_answer 35) Lenzs law of electromagnetic induction corresponds to the
A)
law of conservation of charge
done
clear
B)
law of conservation of energy
done
clear
C)
law of conservation of momentum
done
clear
D)
law of conservation of angular momentum
done
clear
View Answer play_arrow
question_answer 36) Which of the following statement is correct?
A)
The rest mass of a stable nucleus is less than the sum of the rest masses of its separated nucleons.
done
clear
B)
The rest mass of a stable nucleus is greater than the sum of the rest masses of its separated nucleons.
done
clear
C)
In nuclear fission, energy is released by fusion two nuclei of medium mass (approximately100 amu).
done
clear
D)
In nuclear fission, energy is released by fragmentation of a very low nucleus.
done
clear
View Answer play_arrow
question_answer 37) A Si and a Ge diode has identical physical dimensions. The band gap in Si is larger than that in Ge. An identical reverse bias is applied across the diodes
A)
The reverse current in Ge is larger than that in Si
done
clear
B)
The reverse current in Si is larger than that in Ge
done
clear
C)
The reverse current is identical in the two diodes
done
clear
D)
The relative magnitude of the reverse currents cannot be determined from the given data only
done
clear
View Answer play_arrow
question_answer 38) A thin wire of resistance \[4\Omega \] is bent to form a circle. The resistance across any diameter is
A)
\[4\Omega \]
done
clear
B)
\[2\Omega \]
done
clear
C)
\[1\,\Omega \]
done
clear
D)
\[8\,\Omega \]
done
clear
View Answer play_arrow
question_answer 39) A battery of e. m. f. E and internal resistance r is connected to an external resistance R the condition for maximum power transfer is
A)
\[r<R\]
done
clear
B)
\[r>R\]
done
clear
C)
\[r=1/R\]
done
clear
D)
\[r=R\]
done
clear
View Answer play_arrow
question_answer 40) The binary number 10111 is equivalent to the decimal number
A)
19
done
clear
B)
31
done
clear
C)
23
done
clear
D)
22
done
clear
View Answer play_arrow
question_answer 41) Which one of the following pairs is obtained on heating ammonium dichromate?
A)
\[{{N}_{2}}\]and\[{{H}_{2}}O\]
done
clear
B)
\[{{N}_{2}}O\]and \[{{H}_{2}}O\]
done
clear
C)
\[N{{O}_{2}}\]and\[{{H}_{2}}O\]
done
clear
D)
NO and \[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 42) Which one of the following processes is used for the manufacture of calcium?
A)
Reduction of CaO with carbon
done
clear
B)
Reduction of CaO with hydrogen
done
clear
C)
Electrolysis of a mixture of anhydrous \[\text{CaC}{{\text{l}}_{\text{2}}}\] and \[\text{KCl}\]
done
clear
D)
Electrolysis of molten \[\text{Ca(OH}{{\text{)}}_{2}}\]
done
clear
View Answer play_arrow
question_answer 43) Composition of azurite mineral is
A)
\[CuC{{O}_{3}}.CuO\]
done
clear
B)
\[Cu{{(HC{{O}_{3}})}_{2}}.Cu{{(OH)}_{2}}\]
done
clear
C)
\[2CuC{{O}_{3}}-Cu{{(OH)}_{2}}\]
done
clear
D)
\[CuC{{O}_{3}}.2Cu{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 44) When KI is added to acidified solution of sodium nitrite, then
A)
NO gas is liberated and \[{{\text{I}}_{\text{2}}}\] is set free
done
clear
B)
\[{{\text{N}}_{2}}\]gas is liberated and HI is produced
done
clear
C)
\[{{\text{N}}_{\text{2}}}\text{O}\]gas is liberated and \[{{\text{I}}_{2}}\] is set free
done
clear
D)
\[{{\text{N}}_{\text{2}}}\]gas is liberated and \[\text{HOI}\]is produced
done
clear
View Answer play_arrow
question_answer 45) \[\text{Fe(OH}{{\text{)}}_{\text{3}}}\]can be separated from \[\text{Al(OH}{{\text{)}}_{3}}\]by the addition of
A)
NaCI solution
done
clear
B)
dil. HCl solution
done
clear
C)
NaOH solution
done
clear
D)
\[\text{N}{{\text{H}}_{\text{4}}}\text{Cl}\]and \[\text{N}{{\text{H}}_{\text{4}}}\text{OH}\]
done
clear
View Answer play_arrow
question_answer 46) Select the incorrect statement among the following.
A)
\[{{\text{O}}_{\text{3}}}\]is used as germicide for purification of air
done
clear
B)
In \[{{O}_{3}},O-O\]bond length is identical with that of molecular oxygen
done
clear
C)
\[{{\text{O}}_{\text{3}}}\]molecule is angular in shape
done
clear
D)
\[{{\text{O}}_{\text{3}}}\]is an oxidising agent
done
clear
View Answer play_arrow
question_answer 47) The brown complex obtained in the detection of nitrate radical is formulated as \[\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{5}}\text{NO }\!\!]\!\!\text{ S}{{\text{O}}_{4}}.\]What is the oxidation number of Fe in this complex?
A)
+1
done
clear
B)
+2
done
clear
C)
+3
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 48) Sodium nitrate on reduction with Zn in presence of NaOH solution produces \[\text{N}{{\text{H}}_{\text{3}}}\text{.}\]Mass of sodium nitrate absorbing Imole of electron will be
A)
7.750
done
clear
B)
10.625
done
clear
C)
8.000
done
clear
D)
9.875
done
clear
View Answer play_arrow
question_answer 49) In transforming 0.01 mole of PbS to \[\text{PbS}{{\text{O}}_{4}},\]the volume of 10 volume \[{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\]required will be
A)
11.2mL
done
clear
B)
22.4 mL
done
clear
C)
33.6 mL
done
clear
D)
44.8 mL
done
clear
View Answer play_arrow
question_answer 50) An unknown element forms an oxide. What will be the equivalent weight of the element if the oxygen content is 20% by weight?
A)
16
done
clear
B)
32
done
clear
C)
8
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 51) \[\text{2N HCl}\] solution will have same molar concentration as a
A)
\[4.0\text{ }N\text{ }{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[0.5\text{ }N\text{ }{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[1\text{ }N\text{ }{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[2\text{ }N\text{ }{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 52) 1 mole of methyl amine on reaction with nitrous acid gives at NTP
A)
1.0 L of nitrogen
done
clear
B)
22.4 L of nitrogen
done
clear
C)
11.2 L of nitrogen
done
clear
D)
5.6 L of nitrogen
done
clear
View Answer play_arrow
question_answer 53) Addition of sodium acetate to 0.1 M acetic acid will cause
A)
increase in pH
done
clear
B)
decrease in pH
done
clear
C)
no change in pH
done
clear
D)
change in pH that cannot be predicted
done
clear
View Answer play_arrow
question_answer 54) The electronic configuration \[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{9}}\]represents a
A)
metal atom
done
clear
B)
non metal atom
done
clear
C)
non-metallic anion
done
clear
D)
metallic cation
done
clear
View Answer play_arrow
question_answer 55) Unusually high boiling point of water is result of
A)
intermolecular hydrogen bonding
done
clear
B)
intramolecular hydrogen bonding
done
clear
C)
both intra and inter molecular hydrogen bonding
done
clear
D)
high specific heat
done
clear
View Answer play_arrow
question_answer 56) In the reaction \[3A\xrightarrow{{}}2B,\] rate of reaction \[+\frac{d(B)}{dt}\] is equal to
A)
\[-\frac{1}{3}\frac{d[A]}{dt}\]
done
clear
B)
\[-\frac{2}{3}\frac{d[A]}{dt}\]
done
clear
C)
\[+\frac{2d[A]}{dt}\]
done
clear
D)
\[-\frac{3}{2}+\frac{d[A]}{dt}\]
done
clear
View Answer play_arrow
question_answer 57) In a given shell the order of screening effect is
A)
\[~s\text{ }>\text{ }p\text{ }>\text{ }d\text{ }>\text{ }f\]
done
clear
B)
\[5\text{ }>\text{ }p\text{ }>\text{ }f\text{ }>\text{ }d\]
done
clear
C)
\[f\text{ }>d>\text{ }p>s\]
done
clear
D)
\[s\text{ }<\text{ }p\text{ }<\text{ }d\text{ }<\text{ }f\]
done
clear
View Answer play_arrow
question_answer 58) A catalyst is a substance which
A)
increases the equilibrium constant of the reaction
done
clear
B)
increases equilibrium concentration of products
done
clear
C)
does not alter the reaction mechanism
done
clear
D)
changes the activation energy of the reaction
done
clear
View Answer play_arrow
question_answer 59) Which of the following expressions gives the de-Broglie relationship?
A)
\[p=\frac{h}{mv}\]
done
clear
B)
\[\lambda =\frac{h}{mv}\]
done
clear
C)
\[\lambda =\frac{h}{mp}\]
done
clear
D)
\[\lambda \,m=\frac{v}{p}\]
done
clear
View Answer play_arrow
question_answer 60) The bond order in \[\text{O}_{2}^{-}\]ion is
A)
2
done
clear
B)
1
done
clear
C)
2.5
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 61) The rms velocity of an ideal gas at constant pressure varies with density as
A)
\[\frac{1}{\sqrt{d}}\]
done
clear
B)
\[d\]
done
clear
C)
\[\sqrt{d}\]
done
clear
D)
\[{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 62) Solubility product of \[\text{Mg(OH}{{\text{)}}_{\text{2}}}\]at ordinary temperature is \[\text{1}\text{.96}\times {{10}^{-11}}.pH\]of a saturated solution of \[\text{Mg(OH}{{\text{)}}_{\text{2}}}\]will be
A)
10.53
done
clear
B)
8.47
done
clear
C)
6.94
done
clear
D)
3.47
done
clear
View Answer play_arrow
question_answer 63) If the volume of the vessel in which the reaction \[2NO+{{O}_{2}}\text{ }\xrightarrow{{}}\text{ }2N{{O}_{2}}\] is occurring is diminished to 1/3rd of its initial volume. The rate of the reaction will be increased by
A)
3 times
done
clear
B)
9 times
done
clear
C)
27 times
done
clear
D)
36 times
done
clear
View Answer play_arrow
question_answer 64) For which reaction change of entropy will be positive?
A)
\[{{H}_{2}}(g)+{{I}_{2}}(g)\rightleftharpoons 2HI(g)\]
done
clear
B)
\[HCl(g)+N{{H}_{3}}(g)\rightleftharpoons N{{H}_{4}}Cl(s)\]
done
clear
C)
\[N{{H}_{4}}N{{O}_{3}}(s)\rightleftharpoons {{N}_{2}}O(g)+2{{H}_{2}}O(g)\]
done
clear
D)
\[MgO(s)+{{H}_{2}}(g)\rightleftharpoons Mg(s)+2{{H}_{2}}O(l)\]
done
clear
View Answer play_arrow
question_answer 65) The product p of the nuclear reaction\[_{92}^{235}U_{0}^{1}n\xrightarrow{{}}p+_{36}^{92}Kr+3_{0}^{1}n,\]is
A)
\[_{56}^{141}Sr\]
done
clear
B)
\[_{56}^{141}La\]
done
clear
C)
\[_{56}^{141}Ba\]
done
clear
D)
\[_{56}^{141}Cs\]
done
clear
View Answer play_arrow
question_answer 66) The freezing point of water is depressed by \[\text{0}\text{.37}{{\,}^{\text{o}}}\text{C}\] in a 0.01 molal NaCI solution. The freezing point of 0.02 molal solution of urea is depressed by
A)
\[0.37{{\,}^{o}}C\]
done
clear
B)
\[0.74{{\,}^{o}}C\]
done
clear
C)
\[0.185{{\,}^{o}}C\]
done
clear
D)
\[0{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 67) Which one of the following gives, on ozonolysis, both aldehydes and ketones?
A)
\[M{{e}_{2}}C=CHMe\]
done
clear
B)
\[M{{e}_{2}}C=CM{{e}_{2}}\]
done
clear
C)
\[MeC{{H}_{2}}-C(Me)=CM{{e}_{2}}\]
done
clear
D)
\[MeCH(Me)-CH=CHMe\]
done
clear
View Answer play_arrow
question_answer 68) Benzoylation of phenol in alkaline medium is known as
A)
Friedel-Crafts reaction
done
clear
B)
Wurtz-Fittig reaction
done
clear
C)
Schotten-Baumann reaction
done
clear
D)
Sabatier Senderens reduction
done
clear
View Answer play_arrow
question_answer 69) Which one of the following compounds is most reactive towards nucleophilic addition?
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[PhCOC{{H}_{3}}\]
done
clear
C)
\[PhCOPh\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) Distillation of acetone with concentrated sulphuric acid gives
A)
diacetone alcohol
done
clear
B)
mesityl oxide
done
clear
C)
mesitylene
done
clear
D)
propene-2-ol
done
clear
View Answer play_arrow
question_answer 71) \[\text{RC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH}\] can be converted to \[\text{RC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH}\] by the following sequence of steps
A)
\[PB{{r}_{3}},KCN,{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[PB{{r}_{3}},KCN,{{H}_{2}}/{{P}^{+}}\]
done
clear
C)
\[KCN,{{H}_{3}}{{O}^{+}}\]
done
clear
D)
\[HCN,PB{{r}_{3}},{{H}_{3}}{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 72) The major product P in the following reaction is\[C{{H}_{3}}-CH=C{{H}_{2}}\xrightarrow[peroxide]{HI}P\]
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}I\]
done
clear
B)
\[C{{H}_{3}}-\underset{\text{I}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
done
clear
C)
\[\underset{\text{I}}{\mathop{\underset{|}{\mathop{C}}\,}}\,{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
D)
\[\underset{\text{I}}{\mathop{\underset{|}{\mathop{C}}\,}}\,{{H}_{2}}-\underset{\text{I}}{\mathop{\underset{|}{\mathop{C}}\,}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 73) Formation of cyanohydrin from a ketone is an example of
A)
electrophilic addition
done
clear
B)
nucleophilic substitution
done
clear
C)
nucleophilic addition
done
clear
D)
electrophilic substitution
done
clear
View Answer play_arrow
question_answer 74) Which of the following will exhibit cis-trans isomerism?
A)
\[C{{H}_{2}}BrC{{H}_{2}}Br\]
done
clear
B)
\[CB{{r}_{3}}C{{H}_{3}}\]
done
clear
C)
\[CHBr=CHBr\]
done
clear
D)
\[CB{{r}_{2}}=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 75) How many primary amines are possible with the formula \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{11}}}\text{N}\]?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 76) The IUPAC name of \[C{{H}_{3}}-CH=CH-C\equiv CH\]is
A)
pent-3-en-l-yne
done
clear
B)
pent-3-en-4-yne
done
clear
C)
pent-2-en-4-yne
done
clear
D)
pent-2-en-3-yne The IUPAC name of given compound is\[\underset{1}{\mathop{C}}\,{{H}_{3}}-\underset{pent\,-2-en-4-yne}{\mathop{\underset{2}{\mathop{C}}\,H=\underset{3}{\mathop{C}}\,H-\underset{4}{\mathop{C}}\,}}\,\equiv \underset{5}{\mathop{C}}\,-H\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following will produce only one product on reduction with\[\text{LiAl}{{\text{H}}_{\text{4}}}\]?
A)
\[C{{H}_{3}}OCOC{{H}_{2}}C{{H}_{3}}\]
done
clear
B)
\[~C{{H}_{3}}C{{H}_{2}}OCOC{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OCOC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}OCOC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 78) Which one of the following pairs gives effervescence with aq. \[\text{NaHC}{{\text{O}}_{\text{3}}}\]? \[\underset{(II)}{\mathop{\underset{C{{H}_{3}}COOC{{H}_{3}}}{\mathop{\underset{(I)}{\mathop{C{{H}_{3}}COCl}}\,}}\,}}\,\] \[\underset{(IV)}{\mathop{\underset{C{{H}_{3}}COOCOC{{H}_{3}}}{\mathop{\underset{(II)}{\mathop{C{{H}_{3}}COC{{H}_{3}}}}\,}}\,}}\,\]
A)
I and II
done
clear
B)
I and IV
done
clear
C)
II and III
done
clear
D)
I and III
done
clear
View Answer play_arrow
question_answer 79) Which of the following acids has the smallest dissociation constant?
A)
\[~C{{H}_{3}}CHFCOOH\]
done
clear
B)
\[~FC{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
C)
\[BrC{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
D)
\[C{{H}_{3}}CHBrCOOH\]
done
clear
View Answer play_arrow
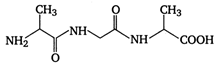
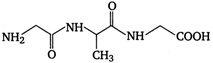
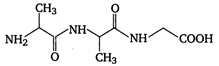
question_answer 80) A tripeptide is written as glycine-alanine - glycine. The correct structure of the tripeptide is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 81) In guava, cucurbits flowers are
A)
hypogynous
done
clear
B)
epigynous
done
clear
C)
perigynous
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 82) In floral formula (K) denotes
A)
polysepalous
done
clear
B)
gamosepalous
done
clear
C)
polypetalous
done
clear
D)
gamopetalous
done
clear
View Answer play_arrow
question_answer 83) Which of the following is ideal for studying meiosis?
A)
Gamete
done
clear
B)
Pollen
done
clear
C)
Microsporangium
done
clear
D)
Root
done
clear
View Answer play_arrow
question_answer 84) To which of the following flower synandrous condition is found?
A)
Sunflower (Helianthus sp)
done
clear
B)
Gourd (Cucurbita sp)
done
clear
C)
Pea (Pisum sativum)
done
clear
D)
Lemon (Citrus sp)
done
clear
View Answer play_arrow
question_answer 85) Which of the following is photophospho-rylarion?
A)
Production of ATP from ADP
done
clear
B)
Production of NADP
done
clear
C)
Synthesis of ADP from ATP
done
clear
D)
Production of PGA
done
clear
View Answer play_arrow
question_answer 86) What is the structural element of cell wall?
A)
Matrix
done
clear
B)
Microtubules
done
clear
C)
Microfibrils
done
clear
D)
Arabinogalactans
done
clear
View Answer play_arrow
question_answer 87) Which chemical is used for induction of polyploidy?
A)
Cytokinin
done
clear
B)
Nitrous acid
done
clear
C)
Colchicine
done
clear
D)
IAA
done
clear
View Answer play_arrow
question_answer 88) In which book, Bentham and Hooker proposed their classification?
A)
Genera Plantarum
done
clear
B)
Species Plantarum
done
clear
C)
Historia Plantarum
done
clear
D)
Historia Naturae
done
clear
View Answer play_arrow
question_answer 89) When does a plant wilt?
A)
When phloem is blocked
done
clear
B)
When xylem is blocked
done
clear
C)
Pith is removed
done
clear
D)
A few leaves are removed
done
clear
View Answer play_arrow
question_answer 90) Which of the following sequence is a correct one foar meiotic cell cycle?
A)
\[{{G}_{1}}\to S\to {{G}_{2}}\to M\to {{G}_{1}}\]
done
clear
B)
\[{{G}_{1}}\to {{G}_{2}}\to M\to {{G}_{2}}\]
done
clear
C)
\[{{G}_{2}}\to {{G}_{1}}\to S\to M\to {{G}_{2}}\]
done
clear
D)
\[~S\to {{G}_{1}}\to {{G}_{2}}\to M\to S\]
done
clear
View Answer play_arrow
question_answer 91) In the following pairs, where do you get lignin in both the element?
A)
Tracheid and collenchyma
done
clear
B)
Sclerenchyma and sieve tube
done
clear
C)
Sclerenchyma and trachea
done
clear
D)
Parenchyma and endodennis
done
clear
View Answer play_arrow
question_answer 92) From which of the following, photosyntheric autotrophs receive their energy?
A)
Heat
done
clear
B)
Inorganic chemicals
done
clear
C)
Organic chemicals
done
clear
D)
Light
done
clear
View Answer play_arrow
question_answer 93) Study of pollen grain is called
A)
Ethmology
done
clear
B)
Palynology
done
clear
C)
Palaeobotany
done
clear
D)
\[\omega \]-raxonomy
done
clear
View Answer play_arrow
question_answer 94) A substance that induces dormancy of seed is
A)
ABA
done
clear
B)
GA
done
clear
C)
thio-urea
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 95) Plants purify air by which process?
A)
Photorespiration
done
clear
B)
Photosynthesis
done
clear
C)
Photophosphorylation
done
clear
D)
Transpiration
done
clear
View Answer play_arrow
question_answer 96) One of the characteristic of sieve tube is
A)
it is a part of phloem
done
clear
B)
function is transport of inorganic solutes
done
clear
C)
it is dead cell
done
clear
D)
sieve plate is not present
done
clear
View Answer play_arrow
question_answer 97) Chromosomes replicate in which stage of meiosis?
A)
Prophase-I
done
clear
B)
Prophase-II
done
clear
C)
Telophase-I
done
clear
D)
Interphase
done
clear
View Answer play_arrow
question_answer 98) Which one of the following conditions in chromosome number is called monosomy?
A)
2n + 1
done
clear
B)
2n + 2
done
clear
C)
2n - 1
done
clear
D)
2n - 2
done
clear
View Answer play_arrow
question_answer 99) Which one of the following statement correctly define the term homonym?
A)
Identical name of two different taxon
done
clear
B)
Two or more names belonging to the same taxon
done
clear
C)
When species name repeats the generic name
done
clear
D)
Other name of a taxon given in a language other than the language of zoological botanical nomenclature
done
clear
View Answer play_arrow
question_answer 100) Which of the following is called stress hormone?
A)
Absdsic add
done
clear
B)
Auxin
done
clear
C)
Cytokinin
done
clear
D)
Gibberellic add
done
clear
View Answer play_arrow
question_answer 101) Dimorphism of diloroplast is found in
A)
\[{{C}_{3}}\]plants
done
clear
B)
\[{{C}_{4}}-\] plants
done
clear
C)
CAM plants
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 102) Which of the following element is responsible for Minamata disease?
A)
Hg
done
clear
B)
Pb
done
clear
C)
Cd
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 103) Shorter generation dme of E. coli compared to eukaryotes may be explained on the basis of
A)
shape
done
clear
B)
large surface : volume ratio
done
clear
C)
presence of cell wall
done
clear
D)
absence of organelles
done
clear
View Answer play_arrow
question_answer 104) Division in a bacterial cell is carried out through
A)
multiple fission
done
clear
B)
binary fission
done
clear
C)
budding
done
clear
D)
plasmotomy
done
clear
View Answer play_arrow
question_answer 105) The Gram (-) bacteria detect and respond to the chemicals in their surroundings by
A)
muramic acid
done
clear
B)
lipopolysaccharide
done
clear
C)
volutin granules
done
clear
D)
porin
done
clear
View Answer play_arrow
question_answer 106) Mesoglia is seen in between
A)
ectoderm and endoderm
done
clear
B)
ectoderm and mesoderm
done
clear
C)
mesoderm and endoderm
done
clear
D)
just below mesoderm
done
clear
View Answer play_arrow
question_answer 107) Green gland is the excretory organ of
A)
prawn
done
clear
B)
butterfly
done
clear
C)
snail
done
clear
D)
earthworm
done
clear
View Answer play_arrow
question_answer 108) The parasite, which completes its life cycle in a single host is
A)
Fasciola hepatica
done
clear
B)
Plasmodium vivax
done
clear
C)
Taenia soiium
done
clear
D)
Ascaris lumbricoides
done
clear
View Answer play_arrow
question_answer 109) Name one disease of mulberry silkworm caused by Protozoa
A)
pebrine
done
clear
B)
graseri
done
clear
C)
flacheri
done
clear
D)
muscardine
done
clear
View Answer play_arrow
question_answer 110) Water vascular system is present in which of the following phylum?
A)
Porifera
done
clear
B)
Cnidaria
done
clear
C)
Ctenophora
done
clear
D)
Echinodennata
done
clear
View Answer play_arrow
question_answer 111) Which one of the following bacterium is used extensively as biopesticide?
A)
Bacillus subtilis
done
clear
B)
Bacillus thuringiensis
done
clear
C)
Streptococcus lactis
done
clear
D)
Lactobacillus acidophilus
done
clear
View Answer play_arrow
question_answer 112) Which one among the following is just a cloning plasmid not an expression plasmid?
A)
pBAD-18-Cam
done
clear
B)
pBCSK
done
clear
C)
pUC18
done
clear
D)
pET
done
clear
View Answer play_arrow
question_answer 113) Royal jelly is secreted from
A)
hypopharyngeal gland
done
clear
B)
salivary gland
done
clear
C)
milk gland
done
clear
D)
integumentary gland
done
clear
View Answer play_arrow
question_answer 114) Uver damage caused by excessive drinking of alcohol is called
A)
hepatitis
done
clear
B)
dematitis
done
clear
C)
liver cirrhosis
done
clear
D)
liver diarrhea
done
clear
View Answer play_arrow
question_answer 115) Pests which only feed and oviposit on crop are called
A)
major pests
done
clear
B)
minor pests
done
clear
C)
accidental pests
done
clear
D)
occasional pests
done
clear
View Answer play_arrow
question_answer 116) In which triploblastic animal, coelom is absent?
A)
Platyhelminthes
done
clear
B)
Asdielnunthes
done
clear
C)
Annelids
done
clear
D)
Arthropoda
done
clear
View Answer play_arrow
question_answer 117) Name one frequently used fungidde
A)
griseonuvin
done
clear
B)
chloramphenicol
done
clear
C)
cycocel
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 118) Pith is absent in
A)
protostele
done
clear
B)
eustele
done
clear
C)
amphiphloic stele
done
clear
D)
ectophloic stele
done
clear
View Answer play_arrow
question_answer 119) Small particles projecting from the inner membranes and cristae of mitochondria are known as
A)
myeloid bodies
done
clear
B)
microsomes
done
clear
C)
infonnosomes
done
clear
D)
oxysomes
done
clear
View Answer play_arrow
question_answer 120) In which of the following phyla, compound eyes are present?
A)
Annelida
done
clear
B)
Arthropoda
done
clear
C)
Mollusca
done
clear
D)
Echinodennata
done
clear
View Answer play_arrow
question_answer 121) Seed habit originated in
A)
Bryophyta
done
clear
B)
Pteridophyta
done
clear
C)
Gymnosperms
done
clear
D)
Angiosperms
done
clear
View Answer play_arrow
question_answer 122) Which one of the following is the infective stage of Ascaris lumbricoides?
A)
Unsegmented egg
done
clear
B)
Egg with first stage larva
done
clear
C)
Egg with second stage larva
done
clear
D)
Free third stage larva
done
clear
View Answer play_arrow
question_answer 123) The father of green revolution in India is
A)
Calvin
done
clear
B)
Haberiandt
done
clear
C)
Norman Boriaug
done
clear
D)
Swaminathan
done
clear
View Answer play_arrow
question_answer 124) In which stage of its life cycle, the silk moth begins to produce silk fibre?
A)
3rd instar larva
done
clear
B)
4th instar larva
done
clear
C)
5th instar larva
done
clear
D)
Pupa
done
clear
View Answer play_arrow
question_answer 125) The vector of the kala- azar is
A)
Aedes sp
done
clear
B)
Anopheles stephenis
done
clear
C)
Culex fatigans
done
clear
D)
Phlebotomus sp
done
clear
View Answer play_arrow
question_answer 126) The resolving power of a compound light microscope will be greatest if the source of light is
A)
blue
done
clear
B)
green
done
clear
C)
yellow
done
clear
D)
red
done
clear
View Answer play_arrow
question_answer 127) Which of the following be named for DNA produced from RNA?
A)
A-DNA
done
clear
B)
B-DNA
done
clear
C)
C-DNA
done
clear
D)
Z-DNA
done
clear
View Answer play_arrow
question_answer 128) Which of the following is not a Green-house gas?
A)
\[{{N}_{2}}O\]
done
clear
B)
\[CFC\]
done
clear
C)
\[{{O}_{3}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 129) India became a party to Convention on biological diversity in the year
A)
1994
done
clear
B)
1993
done
clear
C)
1992
done
clear
D)
1988
done
clear
View Answer play_arrow
question_answer 130) Increase of BOD in water leads to
A)
increase in the dissolved \[{{O}_{2}}\] concentration
done
clear
B)
decrease in the dissolved \[{{O}_{2}}\]concentration
done
clear
C)
maintenance of dissolved \[{{O}_{2}}\]concentration at the same level
done
clear
D)
no effect on dissolved \[{{O}_{2}}\]concentration
done
clear
View Answer play_arrow
question_answer 131) Freon gas causing stratospheric \[{{O}_{3}}\]depletion is mainly released from
A)
refrigerator
done
clear
B)
automobile
done
clear
C)
thermal power plant
done
clear
D)
steel industry
done
clear
View Answer play_arrow
question_answer 132) The equation \[\frac{\Delta {{N}_{n}}}{N\Delta t}\]represents, which of the following?
A)
Natality
done
clear
B)
Growth ray
done
clear
C)
Mortality
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 133) Identify which one of the following is an example, of incomplete ecosystem?
A)
Grassland
done
clear
B)
Cave
done
clear
C)
River
done
clear
D)
Wetland
done
clear
View Answer play_arrow
question_answer 134) Which of the following hormone helps in secretion of HCl from stomach?
A)
Renin
done
clear
B)
Gastrin
done
clear
C)
Secretin
done
clear
D)
Somatomedin
done
clear
View Answer play_arrow
question_answer 135) Liquid, which collects in Bowmans capsule is
A)
water and sulphates
done
clear
B)
water and glycogen
done
clear
C)
plasma minus blood protein
done
clear
D)
concentrated urine
done
clear
View Answer play_arrow
question_answer 136) Diabetes insipidus is caused due to the lack of
A)
ADH
done
clear
B)
ACTH
done
clear
C)
insulin
done
clear
D)
glucagon
done
clear
View Answer play_arrow
question_answer 137) Downs syndrome occurs as a result of
A)
trisomy
done
clear
B)
tetrasomy
done
clear
C)
autopolyploidy
done
clear
D)
allopolyploidy
done
clear
View Answer play_arrow
question_answer 138) Which statement is incorrect for ion-channels?
A)
They are proteins
done
clear
B)
Movement through them is simple diffusion
done
clear
C)
Movement through them is from high to low concentration
done
clear
D)
All ions pass through the same type of channel
done
clear
View Answer play_arrow
question_answer 139) Quaternary structure of protein
A)
consists of four subunits
done
clear
B)
may be either \[\alpha \]or \[\beta \]
done
clear
C)
is unrelated to two functions of the protein
done
clear
D)
is dictated by the primary structures of the individual subunits
done
clear
View Answer play_arrow
question_answer 140) Primary and secondary active transport both
A)
generate ATP
done
clear
B)
use ATP directly
done
clear
C)
can move solutes against their concentration gradient
done
clear
D)
include the passive movement of glucose molecule.
done
clear
View Answer play_arrow
question_answer 141) Which type of membrane is most abundant within a cell?
A)
ER membrane
done
clear
B)
Nuclear membrane
done
clear
C)
Golgi membrane
done
clear
D)
Plasma membrane
done
clear
View Answer play_arrow
question_answer 142) Only one of the following four ways through which AIDS can spread?
A)
Infected needles and syringes
done
clear
B)
Through mosquito bites
done
clear
C)
Looking after AIDS patient
done
clear
D)
Shaking hands, coughing, sneezing, hugging
done
clear
View Answer play_arrow
question_answer 143) Passive immunity can be obtained through
A)
antigens
done
clear
B)
vaccines
done
clear
C)
antibiotics
done
clear
D)
antibodies
done
clear
View Answer play_arrow
question_answer 144) Pan of the brain concerned with the muscular movement is
A)
cerebellum
done
clear
B)
thalamus
done
clear
C)
Hippocampus
done
clear
D)
temporal lobe of cerebrum
done
clear
View Answer play_arrow
question_answer 145) Active immunity development is related to
A)
natural killer cells
done
clear
B)
memory cells
done
clear
C)
helper T cells
done
clear
D)
suppressor T cells
done
clear
View Answer play_arrow
question_answer 146) In human, corpus callosum connects
A)
the two optic lobes
done
clear
B)
bone and muscle
done
clear
C)
the two cerebral hemispheres
done
clear
D)
two lobes of pituitary gland
done
clear
View Answer play_arrow
question_answer 147) Brocas area of speech is present in
A)
frontal lobe
done
clear
B)
parietal lobe and partially in temporal lobe
done
clear
C)
temporal lobe
done
clear
D)
temporal and occipital lobe
done
clear
View Answer play_arrow
question_answer 148) Filariasis is caused by
A)
dead adult filariae
done
clear
B)
microfilariae
done
clear
C)
biting of filarial worm
done
clear
D)
presence of bacteria in filarial wall
done
clear
View Answer play_arrow
question_answer 149) Identify the sulphur containing ammo add
A)
proline
done
clear
B)
methionine
done
clear
C)
asparric acid
done
clear
D)
tryptophan
done
clear
View Answer play_arrow
question_answer 150) Which of the following statements is related to Starlings law of heart?
A)
Greater the stroke volume greater is the heart rate
done
clear
B)
Greater the initial length of the cardiac muscle fibre, more is the force of contraction of heart
done
clear
C)
Greater the minute volume, greater is the heart rate
done
clear
D)
Lesser the length of cardiac muscle fibre greater is the force of contraction of heart
done
clear
View Answer play_arrow
question_answer 151) Indicate, the inheritance of which of the following is controlled by multiple alleles?
A)
Colourblindness
done
clear
B)
Sickle cell anaemia
done
clear
C)
Blood group
done
clear
D)
Phenylketoneuria
done
clear
View Answer play_arrow
question_answer 152) Which of the following conditions is related to haemophilia?
A)
A responsible recessive gene present in the X-chromosome
done
clear
B)
A responsible dominant gene present in the X-chromosome
done
clear
C)
A responsible dominant gene present in the Y-chromosome
done
clear
D)
A responsible dominant gene present in the autosomal chromosome
done
clear
View Answer play_arrow
question_answer 153) The organ in the human body, where glycogenolysis cakes place?
A)
Muscle
done
clear
B)
Liver
done
clear
C)
Small intestine
done
clear
D)
Kidney
done
clear
View Answer play_arrow
question_answer 154) Which of the following carbohydrates is not a disaccharide?
A)
Maltose
done
clear
B)
Lactose
done
clear
C)
Sucrose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 155) The absence of which clotting factor leads to haemophilia - A?
A)
Factor VII
done
clear
B)
Factor VIII
done
clear
C)
Factor K
done
clear
D)
Factor X
done
clear
View Answer play_arrow
question_answer 156) Which of the following vitamins are fat soluble?
A)
A, B, C, K
done
clear
B)
A, B, D, E
done
clear
C)
A, D, E, K
done
clear
D)
A, D, C, K
done
clear
View Answer play_arrow
question_answer 157) In which method of transport, in plasma membrane does not require carrier molecule?
A)
Active transport
done
clear
B)
Facilitated diffusion
done
clear
C)
Simple diffusion
done
clear
D)
\[N{{a}^{+}}-\text{ }{{K}^{+}}\] pump
done
clear
View Answer play_arrow
question_answer 158) Which structure is present in both prokaryotic and eukaryotic plant cells?
A)
Cell wall
done
clear
B)
Nucleus
done
clear
C)
Chloroplast
done
clear
D)
Mitochondria
done
clear
View Answer play_arrow
question_answer 159) Oryza sativa is the binomial name of the rice plant, the sativa stands for
A)
specific name
done
clear
B)
specific epithet
done
clear
C)
species name
done
clear
D)
specific nomenclature
done
clear
View Answer play_arrow
question_answer 160) The first carbon dioxide fixation in \[{{C}_{4}}\] pathway occurs in chloroplasts of
A)
guard cells
done
clear
B)
mesophyll cells
done
clear
C)
bundle sheath cells
done
clear
D)
epidermal cells
done
clear
View Answer play_arrow