question_answer 1) The SI unit of gravitational potential is:
A)
\[J\]
done
clear
B)
\[J-k{{g}^{-1}}\]
done
clear
C)
\[J-kg\]
done
clear
D)
\[J-k{{g}^{2}}\]
done
clear
View Answer play_arrow
question_answer 2) The magnitude of a vector, on the addition of two vectors \[6\vec{i}+7\vec{j}\] and \[3\vec{i}+4\vec{j},\] is :
A)
\[\sqrt{132}+\]
done
clear
B)
\[\sqrt{136}\]
done
clear
C)
\[\sqrt{136}\]
done
clear
D)
\[\sqrt{202}\]
done
clear
View Answer play_arrow
question_answer 3) The potential energy of a particle varies with distance x from a fixed origin as \[V=\left( \frac{A\sqrt{x}}{x+B} \right);\] where A and B are constants. The dimensions of A B are:
A)
\[[M{{L}^{5/2}}{{T}^{-2}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[{{M}^{3/2}}{{L}^{3/2}}{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{7/2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 4) The acceleration experienced by a moving boat after its engine is cut off, is given by \[a=-k{{v}^{3}},\] where k is a constant. If \[{{v}_{0}}\] is the magnitude of velocity at cut off, then the magnitude of the velocity at time t after the cut off is :
A)
\[\frac{{{v}_{0}}}{2ktv_{0}^{2}}\]
done
clear
B)
\[\frac{{{v}_{0}}}{1+2ktv_{0}^{2}}\]
done
clear
C)
\[\frac{{{v}_{0}}}{\sqrt{1-2kv_{0}^{2}}}\]
done
clear
D)
\[\frac{{{v}_{0}}}{\sqrt{1+2kt\,v_{0}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 5) A passenger train is moving at \[5\,\text{m}{{\text{s}}^{-1}}.\] An express train is travelling at \[30\,\text{m}{{\text{s}}^{-1}},\] on the same track and rear side of the passenger tsrain at some distance. The driver in express train applied brakes to avoid collision. If the retardation due to brakes is \[4\,\text{m}{{\text{s}}^{-2}},\] the time in which the accident is avoided after the application of brakes is :
A)
4.25 s
done
clear
B)
5.25 s
done
clear
C)
6.25 s
done
clear
D)
7.25 s
done
clear
View Answer play_arrow
question_answer 6) A weightless thread can bear tension upto 37 N. A stone of mass 500 g is tied to it and revolved in a circular path of radius 4 m in a vertical plane. If \[g=10\,\text{m}{{\text{s}}^{-2}},\] then the maximum angular velocity of the stone will be:
A)
\[2\,\text{rad}\,{{s}^{-1}}\]
done
clear
B)
\[4\,\text{rad}\,{{s}^{-1}}\]
done
clear
C)
\[8\,\text{rad}\,{{s}^{-1}}\]
done
clear
D)
\[16\,\text{rad}\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
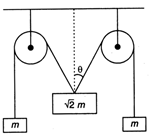
question_answer 7)
The pulleys and strings shown in the figure are smooth and of negligible mass. For the system to remain in equilibrium, the angle \[\theta \] should be:
A)
\[{{0}^{o}}\]
done
clear
B)
\[{{30}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{60}^{o}}\]
done
clear
View Answer play_arrow
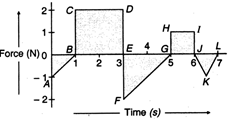
question_answer 8)
A force-time graph for a linear motion of a body is shown in the figure. The change in linear momentum between 0 and 7 s is :
A)
\[2\,N-s\]
done
clear
B)
\[3\,N-s\]
done
clear
C)
\[4\,N-s\]
done
clear
D)
\[5\,N-s\]
done
clear
View Answer play_arrow
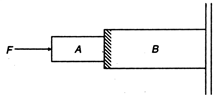
question_answer 9)
Figure shows two blocks A and B pushed against the smooth wall with the force F. The wall is smooth but the surfaces in contact of A and B are rough. Which of the following is true for the system of blocks?
A)
The system can not be in equilibrium
done
clear
B)
F should be equal to weight of A and B
done
clear
C)
F should be less than the weight of A and B
done
clear
D)
F should be more than the weight of A and B
done
clear
View Answer play_arrow
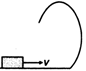
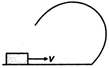
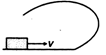
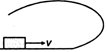
question_answer 10) A small block is shot into each of the four tracks as shown below. Each of the frictionless track rises to the same height. The speed, with which the block enters the tracks, is same in all cases. At the highest point of the track, normal reaction is maximum in :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
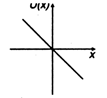
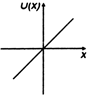
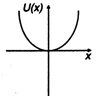
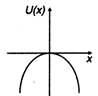
question_answer 11) A particle is placed at the origin and a force \[F=k\text{ }x\] is acting on it (where k is positive constant). If u (0) = 0, the graph of \[u(x)\] versus \[x\] will be (where u is potential energy function) :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 12)
Two blocks of equal masses m are released from the top of a smooth fixed wedge as shown in the figure. The acceleration of the centre of mass of the two blocks is :
A)
g
done
clear
B)
\[\frac{g}{2}\]
done
clear
C)
\[\frac{3g}{4}\]
done
clear
D)
\[\frac{g}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 13) The moment of inertia of a uniform circular disc of radius R and mass M about an axis passing from the edge of the disc and normal to the disc is :
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{1}{2}M{{R}^{2}}\]
done
clear
C)
\[\frac{3}{2}M{{R}^{2}}\]
done
clear
D)
\[\frac{7}{2}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 14) Two spherical bodies of mass M and 5M and radii R and 2R respectively are released in free space with initial separation between their centres equal to 12 R. If they attract each other due to gravitational force only, then the distance covered by the smaller body just before collision, is :
A)
1.5R
done
clear
B)
2.5R
done
clear
C)
\[4.5\,R\]
done
clear
D)
7.5 R
done
clear
View Answer play_arrow
question_answer 15) A satellite orbiting the earth in a circular orbit of radius R completes one revolution in 3 h. If orbital radius of geostationary satellite is 36,000 km, orbital radius of earth is :
A)
6000 km
done
clear
B)
9000 km
done
clear
C)
12000 km
done
clear
D)
15000 km
done
clear
View Answer play_arrow
question_answer 16) A wire can be broken by applying a load of 200 N. The force required to break another wire of the same length and same material, but double in diameter, is :
A)
200 N
done
clear
B)
400 N
done
clear
C)
600 N
done
clear
D)
800 N
done
clear
View Answer play_arrow
question_answer 17) A cube of side 40 mm has its upper face displaced by 0.1 mm by a tangential force of 8 kN. The shearing modulus of cube is :
A)
\[2\times {{10}^{9}}\,N{{m}^{-2}}\]
done
clear
B)
\[4\times {{10}^{9}}\,N{{m}^{-2}}\]
done
clear
C)
\[8\times {{10}^{9}}\,N{{m}^{-2}}\]
done
clear
D)
\[16\times {{10}^{9}}\,N{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 18) The neck and bottom of a bottle are 3 cm and 15 cm in radius respectively. If ±e cork is pressed with a force 12 N in the neck of the bottle, then force exerted on the bottom of the bottle is :
A)
30 N
done
clear
B)
150 N
done
clear
C)
300 N
done
clear
D)
600 N
done
clear
View Answer play_arrow
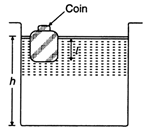
question_answer 19)
A wooden block, with a coin placed on its top, floats in water as shown in the figure. The distances \[h\] and \[l\] are shown there. After sometime, the coin falls into the water. Then :
A)
both \[l\] and h increase
done
clear
B)
both \[l\] and h decrease
done
clear
C)
\[l\] decreases and h increases
done
clear
D)
\[l\] increases and h decreases
done
clear
View Answer play_arrow
question_answer 20) A square wire frame of size L is dipped in a liquid. On taking out a membrane is formed. If the surface tension of liquid is T, then force acting on a frame will be :
A)
2T L
done
clear
B)
4T L
done
clear
C)
8T L
done
clear
D)
16 T L
done
clear
View Answer play_arrow
question_answer 21) A 20 cm long capillary tube is dipped in water. The water rises up to 8 cm. If entire arrangement is put in a freely falling elevator, the length of water column in the capillary tube will be :
A)
4 cm
done
clear
B)
8 cm
done
clear
C)
10cm
done
clear
D)
20 cm
done
clear
View Answer play_arrow
question_answer 22) A metal plate of area \[\text{1}{{\text{0}}^{\text{3}}}\,\text{c}{{\text{m}}^{\text{2}}}\]rests on a layer of oil 6 mm thick. A tangential force of \[{{10}^{-2}}\,\text{N}\]is applied on it to move it with a constant velocity of \[6\,\text{cm}{{\text{s}}^{-1}}.\] The coefficient of viscosity of the liquid is :
A)
0.1 poise
done
clear
B)
0.5 poise
done
clear
C)
0.7 poise
done
clear
D)
0.9 poise
done
clear
View Answer play_arrow
question_answer 23) Sixty four spherical rain drops of equal size are falling vertically through air with a terminal velocity \[1.5\,\text{m}{{\text{s}}^{-1}}.\] If these drops coalesce to form a big spherical drop, then terminal velocity of big drop is :
A)
\[8\,m{{s}^{-1}}\]
done
clear
B)
\[16\,m{{s}^{-1}}\]
done
clear
C)
\[24\,m{{s}^{-1}}\]
done
clear
D)
\[32\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 24) A cylinder of height 20 m is completely filled with water. The velocity of efflux of water (in \[\text{m}{{\text{s}}^{-1}}\]through a hole on the side wall of the cylinder near its bottom, is :
A)
10
done
clear
B)
20
done
clear
C)
25.5
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 25) A liquid flows through a pipe of non-uniform cross-section. If \[{{\text{A}}_{\text{1}}}\]and \[{{\text{A}}_{2}}\]arc the cross-sectional areas of the pipe at two points, the ratio of velocities of the liquid at these points will be :
A)
\[{{A}_{1}}{{A}_{2}}\]
done
clear
B)
\[\frac{{{A}_{1}}}{{{A}_{2}}}\]
done
clear
C)
\[\frac{{{A}_{2}}}{{{A}_{1}}}\]
done
clear
D)
\[\frac{1}{{{A}_{1}}{{A}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 26) A particle executes simple harmonic motion between \[x=-A\]and \[x=+A.\]The time taken for it to go from 0 to A/2 is \[{{T}_{1}}\]and to go from A/2 to A is \[{{T}_{2}}.\]Then :
A)
\[{{T}_{1}}<{{T}_{2}}\]
done
clear
B)
\[{{T}_{1}}>{{T}_{2}}\]
done
clear
C)
\[{{T}_{1}}={{T}_{2}}\]
done
clear
D)
\[{{T}_{1}}=2{{T}_{2}}\]
done
clear
View Answer play_arrow
question_answer 27) A body executes simple harmonic motion. The potential energy (PE), kinetic energy (KE) and total energy (TE) are measured as a function of displacement y. Which of the following statements is true?
A)
TE is zero when \[x=0\]
done
clear
B)
PE is maximum when \[x=0\]
done
clear
C)
KE is maximum when \[x=0\]
done
clear
D)
KE is maximum when \[x\] is maximum
done
clear
View Answer play_arrow
question_answer 28) If mass-energy equivalence is taken into account, when water is cooled to form ice, the mass of water should
A)
increase
done
clear
B)
remain unchanged
done
clear
C)
decrease
done
clear
D)
first increase then decrease
done
clear
View Answer play_arrow
question_answer 29) The equation of a wave is \[y=5\sin \left( \frac{t}{0.04}-\frac{x}{4} \right);\] where \[x\] is in cm and t in seconds. The maximum velocity of the wave will be :
A)
\[1\,\text{m}{{\text{s}}^{-1}}\]
done
clear
B)
\[2\,\text{m}{{\text{s}}^{-1}}\]
done
clear
C)
\[1.5\,\text{m}{{\text{s}}^{-1}}\]
done
clear
D)
\[1.25\,\text{m}{{\text{s}}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 30) When the two waves \[{{y}_{1}}=a\sin \omega t\]and V \[{{y}_{2}}=a\cos \omega t\] are superimposed, then resultant amplitude is:
A)
a
done
clear
B)
\[2a\]
done
clear
C)
\[\sqrt{2}a\]
done
clear
D)
\[\frac{a}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 31) A train moves towards a stationary observer with speed \[34\,\text{m}{{\text{s}}^{-1}}.\] The train sounds a whistle and its frequency registered by the observer is \[{{v}_{1}}.\]If the trains speed is reduced to \[17\,\text{m}{{\text{s}}^{-1}},\] the frequency registered is \[{{v}_{2}}.\] If the speed of sound is \[340\,\text{m}{{\text{s}}^{-1}},\] then the ratio \[{{v}_{1}}/{{v}_{2}}\]is:
A)
2
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{18}{19}\]
done
clear
D)
\[\frac{19}{18}\]
done
clear
View Answer play_arrow
question_answer 32) Two vibrating strings of the same material but lengths L and 2L have radii 2r and r respectively. They are stretched under the same tension. Both the strings vibrate in their fundamental modes, the one of length L with frequency \[{{v}_{1}}\]and the other with frequency \[{{v}_{2}}.\]The ratio \[{{v}_{1}}/{{v}_{2}}\]is :
A)
2
done
clear
B)
4
done
clear
C)
8
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 33) The temperature, at which centigrade and Fahrenheit scales give the same reading, is:
A)
\[~-\,{{40}^{o}}\]
done
clear
B)
\[~{{40}^{o}}\]
done
clear
C)
\[~-\,{{30}^{o}}\]
done
clear
D)
\[~\,{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 34) A brass rod of length 500 mm and diameter 3 mm is joined to a steel rod of same length and diameter at \[\text{50}{{\,}^{\text{o}}}\text{C}\text{.}\]If the coefficients of linear expansion of brass and steel are \[2.5\times {{10}^{-5}}{{\,}^{o}}{{C}^{-1}}\]and \[1.25\times {{10}^{-5}}{{\,}^{o}}{{C}^{-1}},\] then change in length of the combined rod at \[200{{\,}^{o}}C\]is :
A)
2.4 mm
done
clear
B)
2.8 mm
done
clear
C)
3.2 mm
done
clear
D)
3.6 mm
done
clear
View Answer play_arrow
question_answer 35) A copper block of mass 4 kg is heated in a fumance to a temperature \[\text{425}{{\,}^{\text{o}}}\text{C}\]and then placed on a large ice block. The mass of ice that will melt in this process will be (Specific heat of copper\[=500\,J\,k{{g}^{-1}}-{{\,}^{o}}{{C}^{-1}}\] and heat of fusion of ice\[=336\,kJ\,k{{g}^{-1}}\]):
A)
0.5 kg
done
clear
B)
1 kg
done
clear
C)
1.5 kg
done
clear
D)
2.5 kg
done
clear
View Answer play_arrow
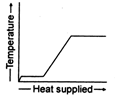
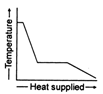
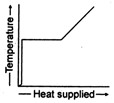
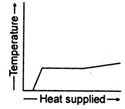
question_answer 36) A block of ice at \[-\text{10}{{\,}^{\text{o}}}\text{C}\]is slowly heated and converted to steam at \[\text{100}{{\,}^{\text{o}}}\text{C}\text{.}\]Which of the following curves represents this phenomenon qualitatively?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 37) The pressure and density of a diatomic gas\[\left( \gamma =\frac{7}{5} \right)\] change adiabatically from \[({{P}_{1}},{{\rho }_{1}})\] to\[({{P}_{2}}{{\rho }_{2}}).\] IF \[\frac{{{\rho }_{2}}}{{{\rho }_{1}}}=32,\]then \[\frac{{{P}_{2}}}{{{P}_{1}}}\]should be:
A)
16
done
clear
B)
32
done
clear
C)
64
done
clear
D)
128
done
clear
View Answer play_arrow
question_answer 38) One mole of an ideal gas at an initial temperature of T K does 6R joules of work adiabatically. If the ratio of specific heats of this gas at constant pressure and at constant volume is 5/3, then final temperature of the gas will be :
A)
(T - 4) K
done
clear
B)
(T + 4) K
done
clear
C)
(T - 2.4) K
done
clear
D)
(T + 2.4) K
done
clear
View Answer play_arrow
question_answer 39) In which mode Of transmission, the heat waves travel along Straight line with the speed of light?
A)
thermal radiation
done
clear
B)
forced convection
done
clear
C)
natural convection
done
clear
D)
thermal conduction
done
clear
View Answer play_arrow
question_answer 40) Consider a compound slab consisting of two different materials having equal lengths, thicknesses and thermal conductivities K and 2K respectively. The equivalent thermal conductivity of the slab is :
A)
\[\sqrt{2}K\]
done
clear
B)
\[3K\]
done
clear
C)
\[\frac{4}{3}K\]
done
clear
D)
\[\frac{2}{3}K\]
done
clear
View Answer play_arrow
question_answer 41) An ideal black body at room temperature is thrown into a fumance. It is observed that:
A)
it is the darkest body at all times
done
clear
B)
it cannot be distinguished at all times
done
clear
C)
initially it is the darkest body and later it becomes brightest
done
clear
D)
initially it is the darkest body and later it cannot be distinguished
done
clear
View Answer play_arrow
question_answer 42) If temperature of the sun were to increase from T to 2 T and its radius from R to 2 R, then how many times the radiant energy will be received on the earth?
A)
4
done
clear
B)
16
done
clear
C)
32
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 43) A black body has a wavelength of\[\lambda \] at temperature 2000 K. Its corresponding wavelength at temperature 3000 K will be :
A)
\[\frac{2\lambda }{3}\]
done
clear
B)
\[\frac{3\lambda }{2}\]
done
clear
C)
\[\frac{4\lambda }{9}\]
done
clear
D)
\[\frac{9\lambda }{4}\]
done
clear
View Answer play_arrow
question_answer 44) According to first law of thermodynamics:
A)
energy is conserved
done
clear
B)
mass is conserved
done
clear
C)
heat is constant in isothermal process
done
clear
D)
heat neither enters nor leaves system First law of thermodynamics is equivalent to conservation of energy.
done
clear
View Answer play_arrow
question_answer 45) Ten moles of an ideal gas at constant temperature 500 K is compressed from 50 L to 5 L. Work done in the process is (Given, \[R=8.31\,J-mo{{l}^{-1}}-{{K}^{-1}}\]) :
A)
\[-1.2\times {{10}^{4}}J\]
done
clear
B)
\[-2.4\times {{10}^{4}}J\]
done
clear
C)
\[-\,4.8\times {{10}^{4}}J\]
done
clear
D)
\[-\,9.6\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 46) A plane mirror produces a magnification of:
A)
zero
done
clear
B)
-1
done
clear
C)
+1
done
clear
D)
between 0 and +1
done
clear
View Answer play_arrow
question_answer 47) In a pond of water, a flame is held 2 m above the surface of water. A fish is at depth of 4 m from water surface. Refractive index of warer is \[\frac{4}{3}.\] The apparent height of the flame from the eyes of fish is :
A)
5.5 m
done
clear
B)
6 m
done
clear
C)
\[\frac{8}{3}m\]
done
clear
D)
\[\frac{20}{3}m\]
done
clear
View Answer play_arrow
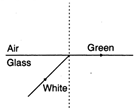
question_answer 48)
White light is incident on the interface of glass and air, as shown in the figure. If green light is just totally and internally reflected, then the emerging ray in air contains :
A)
all colours
done
clear
B)
yellow, orange and red
done
clear
C)
violet, indigo and blue
done
clear
D)
all colours except green
done
clear
View Answer play_arrow
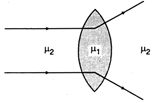
question_answer 49)
A convex lens made up of a material of refractive index \[{{\mu }_{1}}\]is immersed in a medium of refractive index \[{{\mu }_{2}}\]as shown in the figure. The relation between \[{{\mu }_{1}}\]and\[{{\mu }_{2}}\] is :
A)
\[{{\mu }_{1}}<{{\mu }_{2}}\]
done
clear
B)
\[{{\mu }_{1}}>{{\mu }_{2}}\]
done
clear
C)
\[{{\mu }_{1}}={{\mu }_{2}}\]
done
clear
D)
\[{{\mu }_{1}}=\sqrt{{{\mu }_{2}}}\]
done
clear
View Answer play_arrow
question_answer 50) If a flint lens glass of dispersive power 0.066 renders achromatic to a convex lens of crown glass of focal length 60 cm and dispersive power 0.033, then its focal length is:
A)
- 60 cm
done
clear
B)
+ 60 cm
done
clear
C)
-120 cm
done
clear
D)
+ 120cm
done
clear
View Answer play_arrow
question_answer 51) Two coherent light beams of intensity \[I\] and \[4I\]are superposed. The maximum and minimum possible intensities in the resulting beam are :
A)
\[5I\]and \[I\]
done
clear
B)
\[5I\]and\[3I\]
done
clear
C)
\[9I\] and \[I\]
done
clear
D)
\[9I\]and \[3I\]
done
clear
View Answer play_arrow
question_answer 52) If Youngs double slit experiment is performed in water instead of air, then :
A)
no fringes would be seen
done
clear
B)
fringe width would decrease
done
clear
C)
fringe width would increase
done
clear
D)
fringe width would remain unchanged
done
clear
View Answer play_arrow
question_answer 53) In Youngs double-slit experiment, the angular width of fringe formed on a distance screen is \[\text{0}\text{.}{{\text{1}}^{\text{o}}}\text{.}\] If wavelength of light is \[\text{6000}\,\overset{\text{o}}{\mathop{\text{A}}}\,,\] then spacing between the slits is :
A)
\[3.4\times {{10}^{-4}}\,m\]
done
clear
B)
\[4.3\times {{10}^{-4}}m\]
done
clear
C)
\[5.4\times {{10}^{-4}}m\]
done
clear
D)
\[6.3\times {{10}^{-4}}m\]
done
clear
View Answer play_arrow
question_answer 54) A person uses spectacles of power + 2 D. He is suffering from:
A)
myopia
done
clear
B)
presbyopia
done
clear
C)
astigmatism
done
clear
D)
hypermetropia
done
clear
View Answer play_arrow
question_answer 55) The magnification produced by the objective lens and the eye lens of a compound microscope are 25 and 6 respectively. The magnification of this microscope is:
A)
25
done
clear
B)
50
done
clear
C)
150
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 56) Two charged spheres separated by a distance d exert some force (F) on each other. If they are immersed in a liquid of dielectric constant 4, then what is the force exerted, if all other conditions are same?
A)
2F
done
clear
B)
4F
done
clear
C)
\[\frac{F}{2}\]
done
clear
D)
\[\frac{F}{4}\]
done
clear
View Answer play_arrow
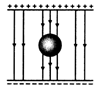
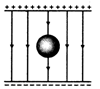
question_answer 57) An uncharged sphere of metal is placed inside a charged parallel plate capacitor. The electric lines of force will look like:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 58) The electric potential at a point on the axis of an electric dipole depends upon the distance \[r(>>a)\] of the point from centre of the dipole as:
A)
\[\propto r\]
done
clear
B)
\[\propto \frac{1}{r}\]
done
clear
C)
\[\propto \frac{1}{{{r}^{2}}}\]
done
clear
D)
\[\propto \frac{1}{{{r}^{3}}}\]
done
clear
View Answer play_arrow
question_answer 59) A charge q is located at the centre of a cube. The electric flux through any face is :
A)
\[\frac{4\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
B)
\[\frac{\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
C)
\[\frac{q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
D)
\[\frac{2\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
View Answer play_arrow
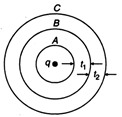
question_answer 60)
Figure shows three spherical and equipotential surfaces A, B and C round a point charge q. The potential difference \[{{V}_{A}}-{{V}_{B}}={{V}_{B}}-{{V}_{C}}.\]If \[{{t}_{1}}\]and \[{{t}_{2}}\] be the distance between them. Then:
A)
\[{{t}_{1}}={{t}_{2}}\]
done
clear
B)
\[{{t}_{1}}>{{t}_{2}}\]
done
clear
C)
\[{{t}_{1}}<{{t}_{2}}\]
done
clear
D)
\[{{t}_{1}}\le {{t}_{2}}\]
done
clear
View Answer play_arrow
question_answer 61) Three capacitors of capacitances \[1\,\mu F,2\mu F\]and 4uF are connected first in a series combination, and then in a parallel combination. The ratio of their equivalent capacitances will be :
A)
2: 49
done
clear
B)
49:2
done
clear
C)
4 : 49
done
clear
D)
49 : 4
done
clear
View Answer play_arrow
question_answer 62) A fully charged capacitor has a capacitance C. It is discharged through a small coil of resistance wire embedded in a thermally insulated block of specific heat capacity s and mass m. If temperature of the block is raised by\[\Delta T,\] the potential difference V across the capacitor is :
A)
\[\frac{ms\Delta T}{C}\]
done
clear
B)
\[\frac{mC\Delta T}{s}\]
done
clear
C)
\[\sqrt{\frac{2mC\Delta T}{s}}\]
done
clear
D)
\[\sqrt{\frac{2ms\Delta T}{C}}\]
done
clear
View Answer play_arrow
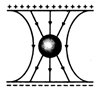
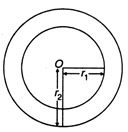
question_answer 63)
In the given figure, a hollow spherical capacitor is shown. The electric field will not be zero at
A)
\[r<{{r}_{1}}\]
done
clear
B)
\[{{r}_{1}}<{{r}_{2}}\]
done
clear
C)
\[r<{{r}_{2}}\]
done
clear
D)
\[{{r}_{1}}<r<{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) At room temperature, copper has free electron density of \[8.4\times {{10}^{28}}{{m}^{-3}}.\] The electron drift velocity in a copper conductor of cross-sectional area of \[{{10}^{-6}}{{m}^{2}}\]and carrying a current of 5.4 A, will be :
A)
\[4\,m{{s}^{-1}}\]
done
clear
B)
\[0.4\,m{{s}^{-1}}\]
done
clear
C)
\[4\,cm\,{{s}^{-1}}\]
done
clear
D)
\[0.4\,mm\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
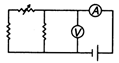
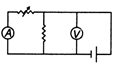
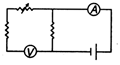
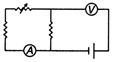
question_answer 65) Which of the following set up can be used to verify the Ohms law?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 66) A uniform wire of resistance R and length L is cut into four equal parts, each of length L/4, which are then connected in parallel combination. The effective resistance of the combination will be:
A)
R
done
clear
B)
4R
done
clear
C)
\[\frac{R}{4}\]
done
clear
D)
\[\frac{R}{16}\]
done
clear
View Answer play_arrow
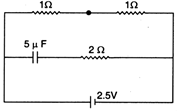
question_answer 67)
A capacitor of capacitance \[\text{5 }\!\!\mu\!\!\text{ F}\]is connected as shown in the figure. The internal resistance of the cell is \[0.5\Omega .\] The amount of charge on the capacitor plates is :
A)
\[8\mu C\]
done
clear
B)
\[40\mu C\]
done
clear
C)
\[20\mu C\]
done
clear
D)
\[10\mu C\]
done
clear
View Answer play_arrow
question_answer 68) Two identical cells of the same emf and sameinternal resistance give the same currentthrough an external resistance \[2\Omega ,\] regardlessof whether they are connected in series orparallel. The internal resistance of the cell is :
A)
\[1\,\Omega \]
done
clear
B)
\[2\,\Omega \]
done
clear
C)
\[3\,\Omega \]
done
clear
D)
\[4\,\Omega \]
done
clear
View Answer play_arrow
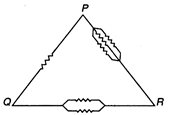
question_answer 69)
Six equal resistance are connected betweenpoints P, Q and R as shown in the figure. Thenthe net resistance will be maximum between :
A)
P and Q
done
clear
B)
Q and R
done
clear
C)
P and Q
done
clear
D)
only two points
done
clear
View Answer play_arrow
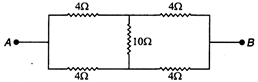
question_answer 70)
The equivalent resistance across A and B is :
A)
\[2\,\Omega \]
done
clear
B)
\[3\,\Omega \]
done
clear
C)
\[4\,\Omega \]
done
clear
D)
\[5\,\Omega \]
done
clear
View Answer play_arrow
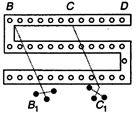
question_answer 71)
For the post office box arrangement todetermine the value of unknown resistance, the unknown resistance should be connected between:
A)
B and C
done
clear
B)
C and D
done
clear
C)
A and D
done
clear
D)
\[{{B}_{1}}\]and\[{{C}_{1}}\]
done
clear
View Answer play_arrow
question_answer 72) An electric current passes through a long straight copper wire. At a distance 5 cm from the straight wire, the magnetic field is B. The magnetic field at 20 cm from the straight wire would be:
A)
\[\frac{B}{6}\]
done
clear
B)
\[\frac{B}{4}\]
done
clear
C)
\[\frac{B}{3}\]
done
clear
D)
\[\frac{B}{2}\]
done
clear
View Answer play_arrow
question_answer 73) In a hydrogen atom, the electron is making\[6.6\times {{10}^{15}}\]rps in a circular path of radius\[\text{0}\text{.53}\,\overset{\text{o}}{\mathop{\text{A}}}\,\text{.}\]What is the magnetic induction produced at the centre of orbit?
A)
6.3 T
done
clear
B)
12.6 T
done
clear
C)
18.9 T
done
clear
D)
25.2 T
done
clear
View Answer play_arrow
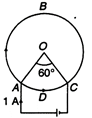
question_answer 74)
A cell is connected between the points A and C of a circular conductor ABCD with 0 as centre and angle \[\text{AOC}\,\text{= 6}{{\text{0}}^{\text{o}}}\text{.}\]If \[{{\text{B}}_{\text{1}}}\]and \[{{\text{B}}_{2}}\]are the magnitudes of the magnetic fields at O due to the currents in ABC and ADC respectively, then ratio \[\frac{{{B}_{1}}}{{{B}_{2}}}\]is :
A)
1
done
clear
B)
2
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 75) An electron of mass m and charge q is travelling with a speed v along a circular path of radius r at right angles to a uniform of magnetic field B. If speed of the electron is doubled and the magnetic field is halved, then resulting path would have a radius of:
A)
\[\frac{r}{4}\]
done
clear
B)
\[\frac{r}{2}\]
done
clear
C)
\[2r\]
done
clear
D)
\[4r\]
done
clear
View Answer play_arrow
question_answer 76) A particle of charge \[-16\times {{10}^{-18}}C\]moving with velocity \[10\,\text{m}{{\text{s}}^{-1}}\] along the \[x-\]axis enters a region where a magnetic field of induction B is along the \[y-\]axis, and an electric field of magnitude \[{{10}^{4}}\,\text{V}{{\text{m}}^{-1}}\] is along the negative\[z-\]axis. If the charged particle continues moving along the \[x-\]axis, then magnitude of B is :
A)
\[{{10}^{-3}}Wb{{m}^{-2}}\]
done
clear
B)
\[{{10}^{3}}Wb{{m}^{-2}}\]
done
clear
C)
\[{{10}^{5}}Wb{{m}^{-2}}\]
done
clear
D)
\[{{10}^{16}}Wb{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 77) The ratio of voltage sensitivity \[({{V}_{S}})\] and current sensitivity \[({{I}_{S}})\] of a moving coil galvanometers:
A)
\[\frac{1}{G}\]
done
clear
B)
\[\frac{1}{{{G}^{2}}}\]
done
clear
C)
\[G\]
done
clear
D)
\[{{G}^{2}}\]
done
clear
View Answer play_arrow
question_answer 78) A galvanometer acting as a voltmeter should have
A)
low resistance in series with its coil
done
clear
B)
low resistance in parallel with its coil
done
clear
C)
high resistance in series with its coil
done
clear
D)
high resistance in parallel with its coil
done
clear
View Answer play_arrow
question_answer 79) In a region, steady and uniform electric and magnetic fields are present. These two fields are parallel to each other. A charged particle is released from rest in this region. The path of the particle will be a :
A)
helix
done
clear
B)
straight line
done
clear
C)
ellipse
done
clear
D)
circle
done
clear
View Answer play_arrow
question_answer 80) The coil in a tangent galvanometer is 16 cm in radius. If a current of 20 mA is to produce a deflection of \[\text{4}{{\text{5}}^{\text{o}}},\] then the number of turns wound on it, is (Take horizontal component of earths magnetic field \[=0.36\times {{10}^{-4}}T\]and\[{{\mu }_{0}}=4\pi \times {{10}^{-7}}\,Wb{{A}^{-1}}{{m}^{-1}}\])
A)
229
done
clear
B)
458
done
clear
C)
689
done
clear
D)
916
done
clear
View Answer play_arrow
question_answer 81) A frog can be levitated in magnetic field produced by a current in a vertical solenoid placed below the frog. This is possible because the body of the frog behaves as:
A)
paramagnetic
done
clear
B)
diamagnetic
done
clear
C)
ferromagnetic
done
clear
D)
anti-ferromagnetic
done
clear
View Answer play_arrow
question_answer 82) The magnetic susceptibility of a material of arod is 499. The absolute permeability of vacuum is \[4\pi \times {{10}^{-7}}\,H{{m}^{-1}},\] The absolute permeability of the material of rod is :
A)
\[\pi \times {{10}^{-4}}\,H{{m}^{-1}}\]
done
clear
B)
\[2\pi \times {{10}^{-4}}\,H{{m}^{-1}}\]
done
clear
C)
\[3\pi \times {{10}^{-4}}\,H{{m}^{-1}}\]
done
clear
D)
\[4\pi \times {{10}^{-4}}\,H{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 83) A small piece of metal wire is dragged across the gap between the poles of a magnet in 0.4 s. If change in magnetic flux in the wire is\[8\times {{10}^{-4}}\text{Wb},\] then emf induced in the wire is:
A)
\[8\times {{10}^{-3}}V\]
done
clear
B)
\[6\times {{10}^{-3}}V\]
done
clear
C)
\[4\times {{10}^{-3}}V\]
done
clear
D)
\[2\times {{10}^{-3}}V\]
done
clear
View Answer play_arrow
question_answer 84) The magnetic flux linked with a coil at any instant t is given by the equation : \[\text{o }\!\!|\!\!\text{ =}\,\text{5}{{\text{t}}^{3}}-100t+300.\]The magnitude of emf induced in the coil after 3 s is :
A)
10 V
done
clear
B)
20 V
done
clear
C)
35 V
done
clear
D)
70 V
done
clear
View Answer play_arrow
question_answer 85) In 0.1 s, the current in a coil increases from 1 A to 1.5 A. If inductance of coil is 60 mH, then induced current in external resistance of \[3\Omega \]will be :
A)
1 A
done
clear
B)
0.5 A
done
clear
C)
0.2A
done
clear
D)
0.1A
done
clear
View Answer play_arrow
question_answer 86) The impedance of a circuit, when a resistance R and an inductor of inductance L are connected in series in an AC circuit of frequency \[f,\] is :
A)
\[\sqrt{R+2{{\pi }^{2}}{{f}^{2}}{{L}^{2}}}\]
done
clear
B)
\[\sqrt{R+4{{\pi }^{2}}{{f}^{2}}{{L}^{2}}}\]
done
clear
C)
\[\sqrt{{{R}^{2}}+4{{\pi }^{2}}{{f}^{2}}{{L}^{2}}}\]
done
clear
D)
\[\sqrt{{{R}^{2}}+2{{\pi }^{2}}{{f}^{2}}{{L}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 87) In a series LCR circuit, resistance \[R=10\,\Omega \]and the impedance \[Z=10\,\Omega .\]The phase difference between the current and the voltage is :
A)
\[{{\text{0}}^{\text{o}}}\]
done
clear
B)
\[\text{3}{{\text{0}}^{\text{o}}}\]
done
clear
C)
\[{{45}^{\text{o}}}\]
done
clear
D)
\[{{60}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 88) A circuit consists of an inductance of 0.5 mH and a capacitor of\[\text{20}\,\text{ }\!\!\mu\!\!\text{ F}\text{.}\] The frequency of the LC oscillations is approximately :
A)
400 Hz
done
clear
B)
88 Hz
done
clear
C)
1600 Hz
done
clear
D)
2400 Hz
done
clear
View Answer play_arrow
question_answer 89) Which of the following electromagnetic waves have the smallest wavelength?
A)
\[\gamma -\]rays
done
clear
B)
X-rays ,
done
clear
C)
UV waves
done
clear
D)
infrared rays
done
clear
View Answer play_arrow
question_answer 90) The kinetic energy of an electron, which isaccelerated in the potential difference of 100V, is:
A)
\[1.6\times {{10}^{-17}}J\]
done
clear
B)
\[1.6\times {{10}^{-14}}J\]
done
clear
C)
\[1.6\times {{10}^{-10}}J\]
done
clear
D)
\[1.6\times {{10}^{-8}}J\]
done
clear
View Answer play_arrow
question_answer 91) J.J. Thomsons cathode ray tube experimentdemonstrated that:
A)
cathode rays are streams of negativelycharged ions
done
clear
B)
all the mass of an atom is essentially in thenucleus
done
clear
C)
the e/m of electrons is much greater thanthee/ mot protons
done
clear
D)
the e/ m ratio of the cathode ray particleschanges when a different gas is placed inthe discharge tube
done
clear
View Answer play_arrow
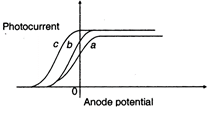
question_answer 92)
The figure shows variation of photocurrentwith anode potential for a photo-sensitivesurface for three different radiations. Let \[{{I}_{a}},{{I}_{b}}\]and \[{{I}_{c}}\]be the intensities and Vg, v^, and v,; be thefrequencies for the curves a,b and crespectively. Then :
A)
\[{{v}_{a}}={{v}_{b}}\]and\[{{I}_{a}}\ne {{I}_{b}}\]
done
clear
B)
\[{{v}_{a}}={{v}_{c}}\]and\[{{I}_{a}}={{I}_{c}}\]
done
clear
C)
\[{{v}_{a}}={{v}_{b}}\]and\[{{I}_{a}}={{I}_{b}}\]
done
clear
D)
\[{{v}_{b}}={{v}_{c}}\]and\[{{I}_{b}}={{I}_{c}}\]
done
clear
View Answer play_arrow
question_answer 93) Wavelength of light emitted from second orbitto first orbit in a hydrogen atom is:
A)
\[\text{6563}\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4102\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4861\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1215\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 94) If M is atomic mass, A is mass number of anucleus, then \[\frac{M-A}{A}\] is called :
A)
mass defect
done
clear
B)
fermi energy
done
clear
C)
binding energy
done
clear
D)
packing fraction
done
clear
View Answer play_arrow
question_answer 95) The half-life of a radio-isotope is 4 h. If initialmass of the isotope was 200 g, then massremaining after 24 h will be :
A)
1.042g
done
clear
B)
2.084 g
done
clear
C)
3.125 g
done
clear
D)
4.167 g
done
clear
View Answer play_arrow
question_answer 96) A radioactive nucleus A finally transforms intoa stable nucleus B. Then A and B can be :
A)
isobars
done
clear
B)
isotones
done
clear
C)
isotopes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 97) If \[{{\,}_{92}}{{U}^{206}}\]emits 8 \[\alpha -\]particles and \[6\beta -\]particles,then the resulting nucleus is:
A)
\[{{\,}_{82}}{{U}^{206}}\]
done
clear
B)
\[{{\,}_{82}}P{{b}^{206}}\]
done
clear
C)
\[{{\,}_{82}}{{U}^{210}}\]
done
clear
D)
\[{{\,}_{82}}{{U}^{214}}\]
done
clear
View Answer play_arrow
question_answer 98) Carbon, silicon and germanium atoms havefour valence electrons each. Their valence andconduction bands are separated by energyband gaps represented by \[{{({{E}_{g}})}_{C}},{{({{E}_{g}})}_{Si}},\] and\[{{({{E}_{g}})}_{Ge}}\]respectively. Which one of the followingrelationships is true in their case ?
A)
\[{{({{E}_{g}})}_{C}}>{{({{E}_{g}})}_{Si}}\]
done
clear
B)
\[{{({{E}_{g}})}_{C}}={{({{E}_{g}})}_{Si}}\]
done
clear
C)
\[{{({{E}_{g}})}_{C}}<{{({{E}_{g}})}_{Ge}}\]
done
clear
D)
\[{{({{E}_{g}})}_{C}}<{{({{E}_{g}})}_{Si}}\]
done
clear
View Answer play_arrow
question_answer 99) Then \[n-\]type semiconductors are obtained,when germanium is doped with :
A)
arsenic
done
clear
B)
phosphorus
done
clear
C)
antimony
done
clear
D)
any one of these
done
clear
View Answer play_arrow
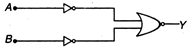
question_answer 100)
Which logic gate is represented by thefollowing combination of logic gates?
A)
OR
done
clear
B)
NOR
done
clear
C)
AND
done
clear
D)
NAND
done
clear
View Answer play_arrow
question_answer 101) Cadmium rods are used for which purpose?
A)
Emit electrons
done
clear
B)
Absorb neutrons
done
clear
C)
Emit neutrons
done
clear
D)
Absorb electrons
done
clear
View Answer play_arrow
question_answer 102) Propionic acid and KOH reacts to produce which one of the following?
A)
Potassium propionate
done
clear
B)
Propyl alcohol
done
clear
C)
Propionaldehyde
done
clear
D)
Does not react
done
clear
View Answer play_arrow
question_answer 103) What is the effect of dilution on the equivalent conductance of strong electrolyte?
A)
Decrease on dilution
done
clear
B)
Remains unchanged
done
clear
C)
Increase on dilution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 104) 35.4 mL of \[\text{HCl}\] is required for the neutralization of a solution containing 0.275 g of sodium hydroxide. The normality of hydrochloric acid is?
A)
0.97 N
done
clear
B)
0.142 N
done
clear
C)
0.194N
done
clear
D)
0.244 N
done
clear
View Answer play_arrow
question_answer 105) When \[{{\text{H}}_{\text{2}}}\text{S}\] gas is passed in a metal sulphate solution in presence of \[\text{N}{{\text{H}}_{\text{4}}}\text{OH,}\] a white precipitate is produced. The metal is identified as:
A)
Zn
done
clear
B)
Fe
done
clear
C)
Pb
done
clear
D)
Hg
done
clear
View Answer play_arrow
question_answer 106) The value of amu is which of the following?
A)
\[1.57\times {{10}^{-24}}kg\]
done
clear
B)
\[1.66\times {{10}^{-24}}kg\]
done
clear
C)
\[1.99\times {{10}^{-23}}kg\]
done
clear
D)
\[1.66\times {{10}^{-27}}kg\]
done
clear
View Answer play_arrow
question_answer 107) The molecule having largest dipole moment among the following is :
A)
\[CH{{I}_{3}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 108) When calcium acetate is distilled, it will produce which of the following compound?
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 109) Which of the following electronic configuration represents noble gas?
A)
\[n{{s}^{2}}n{{p}^{6}}\]
done
clear
B)
\[n{{s}^{2}}n{{p}^{5}}\]
done
clear
C)
\[n{{s}^{2}}n{{p}^{4}}\]
done
clear
D)
\[n{{s}^{2}}n{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 110) Sodium pyrophosphate is represented by which of the following formula?
A)
\[N{{a}_{2}}{{P}_{2}}{{O}_{4}}\]
done
clear
B)
\[N{{a}_{4}}{{P}_{2}}{{O}_{5}}\]
done
clear
C)
\[N{{a}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[N{{a}_{2}}{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 111) Which one of the following can produce hydrogen when treated with metallic sodium?
A)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 112) Which one of the following has unit positive charge and 1 amu mass?
A)
Electron
done
clear
B)
Neutron
done
clear
C)
Proton
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 113) What is the co-ordination number of body centred cube?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 114) Which gas is evolved by the treatment of magnesium with very dilute solution of \[\text{HN}{{\text{O}}_{\text{3}}}\]?
A)
\[~{{N}_{2}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 115) Which of the following compound shows aromatic properties?
A)
Valine
done
clear
B)
Leucine
done
clear
C)
Serine
done
clear
D)
Tyrosine
done
clear
View Answer play_arrow
question_answer 116) Which one of the following pair shows Buffers solution?
A)
\[NaCl+NaOH\]
done
clear
B)
\[C{{H}_{3}}COONa+C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{H}_{3}}COON{{H}_{4}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}+CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 117) \[\text{PC}{{\text{l}}_{\text{3}}}\]and cold water reacts to produce which of the following?
A)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{a2}}\]
done
clear
C)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 118) Which of the following converts carbonyl compounds into hydrocarbons?
A)
\[{{H}_{2}}/Pt\]
done
clear
B)
\[LiAl{{H}_{4}}\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[Zn-Hg/HCl\]
done
clear
View Answer play_arrow
question_answer 119) Which of the following chloride is water insoluble?
A)
\[HCl\]
done
clear
B)
\[AgCl\]
done
clear
C)
Both a and b
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 120) How many neutrons are present in tritium nucleus?
A)
2
done
clear
B)
3
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 121) The total number of protons in \[10g\] of calcium carbonate is \[({{N}_{0}}=6.023\times {{10}^{23}}):\]
A)
\[3.01\times {{10}^{24}}\]
done
clear
B)
\[~4.06\times {{10}^{24}}\]
done
clear
C)
\[2.01\times {{10}^{24}}\]
done
clear
D)
\[3.02\times {{10}^{24}}\]
done
clear
View Answer play_arrow
question_answer 122) By dissolving 5 g substance in 50 g of water, the decraease in freezing point is \[1.2{{\,}^{o}}C.\] The gram molal depression is \[1.85{{\,}^{o}}C.\] The molecular weight of substance is :
A)
105.4
done
clear
B)
118.2
done
clear
C)
137.2
done
clear
D)
154.2
done
clear
View Answer play_arrow
question_answer 123) The high boiling point of water is due to which reason :
A)
co-ordinate bonding
done
clear
B)
covalent bond
done
clear
C)
electrostatic force of attraction
done
clear
D)
hydrogen bonding
done
clear
View Answer play_arrow
question_answer 124) Which is correct for an enothermic reaction?
A)
\[\Delta H\]is positive
done
clear
B)
\[\Delta H\]is negative
done
clear
C)
\[\Delta E\]is negative
done
clear
D)
\[\Delta H=zero\]
done
clear
View Answer play_arrow
question_answer 125) Acetonitriles on hydrolysis produce which of the following?
A)
Amine
done
clear
B)
Acid
done
clear
C)
Amines
done
clear
D)
Carbonyl compounds
done
clear
View Answer play_arrow
question_answer 126) The radius of hydorgen atom is \[0.53\overset{\text{o}}{\mathop{\text{A}}}\,\] The radius of \[{{\,}_{\text{3}}}\text{L}{{\text{i}}^{\text{2+}}}\] is of:
A)
\[1.27\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.17\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.57\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.99\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 127) The purest form of coal is:
A)
peat
done
clear
B)
anthracite
done
clear
C)
bituminous
done
clear
D)
lignite
done
clear
View Answer play_arrow
question_answer 128) The correct sequence of hybridisation of methane, ethene and acetylene is:
A)
\[sp,\text{ s}{{p}^{2}},\text{ s}{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}},\text{ s}{{p}^{3}},\text{ }sp\]
done
clear
C)
\[s{{p}^{3}},\text{ s}{{p}^{2}},\text{ }Sp\]
done
clear
D)
\[s{{p}^{3}},\text{ }Sp,\text{ s}{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 129) Which phosphorus reacts with KOH solution to produce phosphene gas?
A)
White phosphorus
done
clear
B)
Red phosphorus
done
clear
C)
Both and
done
clear
D)
None of Aese
done
clear
View Answer play_arrow
question_answer 130) Saturated fatty acids are represented by which of the formula?
A)
\[{{C}_{n}}{{H}_{n}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{3n}}{{O}_{2}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+1}}\]
done
clear
D)
\[{{C}_{n}}{{H}_{2n}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 131) \[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\xrightarrow{Sn/HCl}{{C}_{6}}{{H}_{5}}X\]X is identified as:
A)
NO
done
clear
B)
\[N{{H}_{2}}\]
done
clear
C)
NHOH
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 132) Acetic acid and \[{{P}_{2}}{{O}_{5}}\]reacts to produce which of the following?
A)
Acetic anhydride
done
clear
B)
Acetaldehyde
done
clear
C)
Phosphoric acid
done
clear
D)
Acetone
done
clear
View Answer play_arrow
question_answer 133) The test for unsaturation is confirmed by the decolourisation of which of the following?
A)
Iodine water
done
clear
B)
\[CuS{{O}_{4}}\]solution
done
clear
C)
Bromine water
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 134) Which of the following element shows maximum valency?
A)
Carbon
done
clear
B)
Barium
done
clear
C)
Nitrogen
done
clear
D)
Sulphur
done
clear
View Answer play_arrow
question_answer 135) The volume of oxygen necessary for the complete combustion of 20 L of propane is :
A)
40 L
done
clear
B)
60 L
done
clear
C)
80 L
done
clear
D)
100 L
done
clear
View Answer play_arrow
question_answer 136) Which reaction is used for the preparation of acetophenone?
A)
Reimer-Tiemann reaction
done
clear
B)
Wurtz-Fittig reaction
done
clear
C)
Friedel-Crafts reaction
done
clear
D)
Cannizaros reaction
done
clear
View Answer play_arrow
question_answer 137) 0.005 M acid solution has 5 pH. The percentage ionisation of acid is :
A)
0.8%
done
clear
B)
0.6%
done
clear
C)
0.4%
done
clear
D)
0.2%
done
clear
View Answer play_arrow
question_answer 138) PVC polymer can be prepared by which of the monomer?
A)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
done
clear
C)
\[~C{{H}_{2}}=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CHCl\]
done
clear
View Answer play_arrow
question_answer 139) \[C{{H}_{3}}COOH\] is weaker acid than \[{{H}_{2}}S{{O}_{4}}.\] It is due to:
A)
more ionization
done
clear
B)
less ionization
done
clear
C)
covalent bond
done
clear
D)
electrovalent bond
done
clear
View Answer play_arrow
question_answer 140) The ortho and para hydrogen differ in respect of which of the following?
A)
In the molecular weight
done
clear
B)
In the nature of spin of protons
done
clear
C)
In the nature of spin of electrons
done
clear
D)
In the number of protons
done
clear
View Answer play_arrow
question_answer 141) Which of the following is correct according to adsorption isotherm?
A)
\[\frac{X}{m}\propto {{p}^{o}}\]
done
clear
B)
\[\frac{X}{m}\propto p\]
done
clear
C)
\[\frac{X}{m}\propto {{p}^{1/n}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 142) \[{{\,}_{\text{84}}}\text{R}{{\text{n}}^{\text{219}}}\] is a member of actinium series. The other member of this series is :
A)
\[{{\,}_{\text{89}}}\text{A}{{\text{c}}^{225}}\]
done
clear
B)
\[{{\,}_{\text{90}}}T{{h}^{232}}\]
done
clear
C)
\[{{\,}_{15}}T{{h}^{35}}\]
done
clear
D)
\[{{\,}_{92}}{{U}^{235}}\]
done
clear
View Answer play_arrow
question_answer 143) The compressibility factor of an ideal gas is :
A)
1
done
clear
B)
2
done
clear
C)
4
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 144) Soaps can be classified as:
A)
carbohydrates
done
clear
B)
ether
done
clear
C)
salts of fatty acids
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 145) In a chemical reaction two reactants takes part. The rate of reaction is directly proportional to the concentration of one of them and inversely proportional to the concentration of the other. The order of reaction is :
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 146) A gaseous mixture contains 56 g of \[{{\text{N}}_{\text{2}}}\text{, 44 g}\] of \[C{{O}_{2}}\]and 16 g of \[C{{H}_{4}}.\]The total pressure of mixture is 720 mm of Hg. The partial pressure of methane is :
A)
75 aim
done
clear
B)
160 atm
done
clear
C)
180 atm
done
clear
D)
215 atm
done
clear
View Answer play_arrow
question_answer 147) The metal used to recover copper from a solution of \[CuS{{O}_{4}}\]is:
A)
Fe
done
clear
B)
He
done
clear
C)
Na
done
clear
D)
Ag
done
clear
View Answer play_arrow
question_answer 148) Amino acids have peptide linkage which is :
A)
\[-CO-NH-\]
done
clear
B)
\[-C-N{{H}_{2}}\]
done
clear
C)
\[SO-NH-\]
done
clear
D)
\[-CO-N-\]
done
clear
View Answer play_arrow
question_answer 149) Phenacetin is used as:
A)
antipyretics
done
clear
B)
antiseptics
done
clear
C)
analgesic
done
clear
D)
antimalarials
done
clear
View Answer play_arrow
question_answer 150) Gravity separation process is used for the concentration of:
A)
calamine
done
clear
B)
haematite
done
clear
C)
chalcopyrite
done
clear
D)
bauxite
done
clear
View Answer play_arrow
question_answer 151) Which of the following is not an ore of magnesium?
A)
Magnesite
done
clear
B)
Dolomite
done
clear
C)
Gypsum
done
clear
D)
Carnallite
done
clear
View Answer play_arrow
question_answer 152) The solubility of \[S{{b}_{2}}{{S}_{3}}\]in water is \[1.0\times {{10}^{-5}}\text{mol/L}\]at 298K. What will be its solubility product?
A)
\[108\times {{10}^{-25}}\]
done
clear
B)
\[1.0\times {{10}^{-25}}\]
done
clear
C)
\[144\times {{10}^{-25}}\]
done
clear
D)
\[126\times {{10}^{-24}}\]
done
clear
View Answer play_arrow
question_answer 153) What will be the pH value of 0.05 M \[Ba{{(OH)}_{2}}\]solution?
A)
12
done
clear
B)
13
done
clear
C)
1
done
clear
D)
12.96
done
clear
View Answer play_arrow
question_answer 154) Which of the following will not react with\[\text{NaOH}\]?
A)
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
D)
\[CH{{(CN)}_{3}}\]
done
clear
View Answer play_arrow
question_answer 155) Industrial name for \[{{H}_{2}}{{S}_{2}}{{O}_{7}}\] is :
A)
Pyrosulphuric acid
done
clear
B)
Marshalls acid
done
clear
C)
Oleum
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 156) \[{{\text{I}}_{\text{2}}}\]dissolves in KI solution due to the formation of:
A)
\[\text{K}{{\text{I}}_{2}}\]and \[{{I}^{-}}\]
done
clear
B)
\[{{K}^{+}},{{I}^{-}}\]and \[{{I}_{2}}\]
done
clear
C)
\[\text{I}_{3}^{-}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) Water gas is :
A)
\[CO+{{N}_{2}}\]
done
clear
B)
\[CO+C{{O}_{2}}+C{{H}_{4}}\]
done
clear
C)
\[C{{O}_{2}}+{{N}_{2}}~\]
done
clear
D)
\[CO+{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 158) Chloroform, when kept open, is oxidised to :
A)
\[C{{O}_{2}}\]
done
clear
B)
\[COC{{l}_{2}}\]
done
clear
C)
\[C{{O}_{2}},\text{ }C{{l}_{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 159) lodoform test is not given by :
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[~{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 160) In Me Arthur forest method, silver is extracted from the solution of \[Na[Ag{{(CN)}_{2}}]\] by the use of:
A)
Fe
done
clear
B)
Mg
done
clear
C)
Cu
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 161) IUPAC name of: \[C{{H}_{3}}C{{H}_{2}}C(Br)=CH-Cl\]is:
A)
2-bromo-l-chloro butane
done
clear
B)
1-chloro-2-bromo butane
done
clear
C)
3-chloro-2-bromo butene-2
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 162) Which of the following elements never show positive oxidation number?
A)
0
done
clear
B)
Fe
done
clear
C)
Ga
done
clear
D)
F
done
clear
View Answer play_arrow
question_answer 163) Which of the following is anhydride of perchloric acid?
A)
\[C{{l}_{2}}{{O}_{7}}\]
done
clear
B)
\[C{{l}_{2}}{{O}_{5}}\]
done
clear
C)
\[C{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[HClO\]
done
clear
View Answer play_arrow
question_answer 164) Quantitative measurement of nitrogen in an organic compound is done by the method:
A)
Berthelot method
done
clear
B)
Belstein method
done
clear
C)
Lassaigne test
done
clear
D)
Kjheldahl method
done
clear
View Answer play_arrow
question_answer 165) Benedicts solution is not reduced by:
A)
formaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
glucose
done
clear
D)
acetic anhydride
done
clear
View Answer play_arrow
question_answer 166) Which of the following sulphides is yellow in colour?
A)
Cus
done
clear
B)
Cds
done
clear
C)
ZnS
done
clear
D)
CoS
done
clear
View Answer play_arrow
question_answer 167) Which of the following element is a metalloid?
A)
Bi
done
clear
B)
Sn
done
clear
C)
Ge
done
clear
D)
C
done
clear
View Answer play_arrow
question_answer 168) Brown ring in the test of \[\text{NO}_{3}^{-}\] is formed due to the formation of:
A)
\[FeS{{O}_{4}}.NO\]
done
clear
B)
\[[Fe{{(S{{O}_{4}})}_{2}}.NO]{{H}_{2}}O\]
done
clear
C)
\[F{{e}_{2}}{{(S{{O}_{4}})}_{3}}.NO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 169) The vapour pressure will be lowest for :
A)
0.1 M sugar solution
done
clear
B)
0.1 M KC1 solution
done
clear
C)
\[0.1\text{ }MCu{{(N{{O}_{3}})}_{2}}\] solution
done
clear
D)
\[~0.1\text{ }M\text{ }AgN{{O}_{3}}\] solution
done
clear
View Answer play_arrow
question_answer 170) Which of the following is a false statement?
A)
Fluorine is more electronegative than chlorine
done
clear
B)
Nitrogen has greater \[\text{I}{{\text{E}}_{\text{1}}}\] than oxygen
done
clear
C)
Lithium is amphoteric
done
clear
D)
Chlorine is an oxidising agent
done
clear
View Answer play_arrow
question_answer 171) What is the name of element with atomic number 105?
A)
Kurchatovium
done
clear
B)
Dubnium
done
clear
C)
Nobelium
done
clear
D)
Holmium
done
clear
View Answer play_arrow
question_answer 172) Which kind of fission is favoured by sunlight?
A)
Heterolytic fission
done
clear
B)
Homolytic fission
done
clear
C)
Both and
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 173) The reagent used in Gateman Koch aldehyde synthesis is :
A)
\[Pb/BaS{{O}_{4}}\]
done
clear
B)
alkaline \[KMn{{O}_{4}}\]
done
clear
C)
acidic \[KMn{{O}_{4}}\]
done
clear
D)
\[CO+HCl\]
done
clear
View Answer play_arrow
question_answer 174) Which type of isomerism is shown by propanal and propanone?
A)
Functional group
done
clear
B)
Metamerism
done
clear
C)
Tautomerism
done
clear
D)
Chain isomerism
done
clear
View Answer play_arrow
question_answer 175) lonisation depends upon:
A)
pressure
done
clear
B)
volume
done
clear
C)
dilution
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 176) Which of the following oxide does not form acidic aqueous solution?
A)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
NO
done
clear
View Answer play_arrow
question_answer 177) Which of the following is a use of alum?
A)
Making explosives
done
clear
B)
Bleaching clothes
done
clear
C)
Water softening
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 178) The first ionisation potential is maximum for:
A)
B
done
clear
B)
N
done
clear
C)
0
done
clear
D)
Be
done
clear
View Answer play_arrow
question_answer 179) Which of the following salt does not get hydrolysed in water?
A)
\[KCl{{O}_{4}}\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[C{{H}_{3}}COONa~\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 180) In the reaction : \[{{H}_{2}}+{{I}_{2}}=2HI\] In a 2 L flask 0.4 moles of each \[{{H}_{2}}\] and \[{{I}_{2}}\] are taken. At equilibrium 0.5 moles of HI are formed. What will be the value of equilibrium constant \[{{K}_{c}}\]?
A)
20.2
done
clear
B)
25.4
done
clear
C)
0.284
done
clear
D)
11.1
done
clear
View Answer play_arrow
question_answer 181) Blood cells will remain as such in :
A)
hypertonic solution
done
clear
B)
hypotonic solution
done
clear
C)
isotonic solution
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 182) Which among the following elements have lowest value of\[\text{I}{{\text{E}}_{\text{1}}}\]?
A)
Pb
done
clear
B)
Sn
done
clear
C)
Si
done
clear
D)
C
done
clear
View Answer play_arrow
question_answer 183) KI and \[\text{CuS}{{\text{O}}_{4}}\] solution when mixed, give :
A)
\[Cu{{l}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}+\text{ }C{{u}_{2}}{{I}_{2}}+{{I}_{2}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}+Cu{{I}_{2}}+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 184) Which of the following is a Lewis base?
A)
\[NaOH\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 185) Wave nature of electrons was demonstrated by :
A)
Schrodinger
done
clear
B)
de-Broglie
done
clear
C)
Davisson and Garmer
done
clear
D)
Heisenberg
done
clear
View Answer play_arrow
question_answer 186) Distribution law was given by :
A)
Henry
done
clear
B)
vant Hoff
done
clear
C)
Nemst
done
clear
D)
Ostwald
done
clear
View Answer play_arrow
question_answer 187) When Cu reacts with \[\text{AgN}{{\text{O}}_{\text{3}}}\]solution, the reaction takes place is :
A)
oxidation of Cu
done
clear
B)
reduction of Cu
done
clear
C)
oxidation of Ag
done
clear
D)
reduction of \[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 188) Cetane is a compound which has very good ignition property. Chemically it is:
A)
\[C{{H}_{3}}{{(C{{H}_{2}})}_{14}}C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}C{{(C{{H}_{2}})}_{11}}C{{H}_{3}}\]
done
clear
C)
\[{{C}_{17}}{{H}_{34}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 189) Aldol condensation will not occur in :
A)
\[HCHO\]
done
clear
B)
\[~C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 190) Which of the following alcohol is used as beverage?
A)
Propanol
done
clear
B)
2-butanol
done
clear
C)
Methanol
done
clear
D)
Ethanol
done
clear
View Answer play_arrow
question_answer 191) The oxidation number of carbon in \[C{{H}_{2}}O\]is :
A)
-2
done
clear
B)
+2
done
clear
C)
0
done
clear
D)
+4
done
clear
View Answer play_arrow
question_answer 192) Which of the following reaction involves oxidation and reduction?
A)
\[NaBr+\text{ }HCl\xrightarrow{{}}\text{ }NaCl+\text{ }HBr\]
done
clear
B)
\[HBr+AgN{{O}_{3}}\xrightarrow{{}}AgBr+HN{{O}_{3}}\]
done
clear
C)
\[{{H}_{2}}+B{{r}_{2}}\xrightarrow{{}}2HBr\]
done
clear
D)
\[N{{a}_{2}}O+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 193) The electrophile involved in the nitration of benzene is :
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{+}\]
done
clear
C)
NO
done
clear
D)
\[NO_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 194)
The correct order of ease of dehydration of following is :
A)
I > II > III
done
clear
B)
III > II > I
done
clear
C)
I > III > II
done
clear
D)
III > I > II
done
clear
View Answer play_arrow
question_answer 195) Fluorine is the best oxidising agent because it has:
A)
highest elecron affinity
done
clear
B)
highest \[E_{red}^{o}\]
done
clear
C)
highest \[E_{oxid}^{o}\]
done
clear
D)
lowest electron affinity
done
clear
View Answer play_arrow
question_answer 196) The bond order of \[\text{O}_{\text{2}}^{\text{+}}\] is the same as in :
A)
\[\text{N}_{2}^{+}\]
done
clear
B)
\[C{{N}^{-}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[N{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 197) When acetamide reacts with \[B{{r}_{2}}\]and caustic soda, then we get:
A)
acetic acid
done
clear
B)
bromoacetic acid
done
clear
C)
methyl amine
done
clear
D)
ethyl amine
done
clear
View Answer play_arrow
question_answer 198) Bleaching action of \[\text{S}{{\text{O}}_{\text{2}}}\]is due to its:
A)
oxidising property
done
clear
B)
acidic property
done
clear
C)
basic property
done
clear
D)
reducing property
done
clear
View Answer play_arrow
question_answer 199) When \[{{\text{I}}_{2}}\] is passed through \[\text{KCl,KF}\]and \[\text{KBr}\]solutions :
A)
\[\text{C}{{\text{l}}_{2}}\]and \[B{{r}_{2}}\]are evolved
done
clear
B)
\[C{{l}_{2}}\]is evolved
done
clear
C)
\[C{{l}_{2}},B{{r}_{2}}\]and \[{{F}_{2}}\]are evolved
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 200) Which of the following dissolves in hot cone. NaOH solution?
A)
Fe
done
clear
B)
Zn
done
clear
C)
Cn
done
clear
D)
Ag
done
clear
View Answer play_arrow
question_answer 201) A prokaryotic cell lacks:
A)
nucleus
done
clear
B)
nuclear membrane
done
clear
C)
membrane bound organelles
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 202) Extranuclear inheritance is a consequence of the presence of genes in:
A)
ER and mitochondria
done
clear
B)
lysosomes and ribosomes
done
clear
C)
ribosomes and chloroplast
done
clear
D)
mitochondria and chloroplasts
done
clear
View Answer play_arrow
question_answer 203) Vesicles of Smooth Endoplasmic Reticulum (SER) are most likely on their way to:
A)
plastids
done
clear
B)
lysosomes
done
clear
C)
nucleolus
done
clear
D)
Golgi apparatus
done
clear
View Answer play_arrow
question_answer 204) Lysosomes are the store house of:
A)
ATP
done
clear
B)
sugar
done
clear
C)
proteins
done
clear
D)
hydrolytic enzymes
done
clear
View Answer play_arrow
question_answer 205) lipids are insoluble in water, because lipid molecules are:
A)
neutral
done
clear
B)
Zwitter ions
done
clear
C)
hydrophobic
done
clear
D)
hydrophilic
done
clear
View Answer play_arrow
question_answer 206) Which of the following is the simplest amino acid?
A)
Glycine
done
clear
B)
Alanine
done
clear
C)
Tyrosine
done
clear
D)
Asparagine
done
clear
View Answer play_arrow
question_answer 207) Carbohydrates, ingested in the diet, are hydrolyzed by the enzyme:
A)
pepsin
done
clear
B)
cellulose
done
clear
C)
a-amylase
done
clear
D)
glycosidase
done
clear
View Answer play_arrow
question_answer 208) Stomach is the site of digestion mainly for:
A)
fats
done
clear
B)
proteins
done
clear
C)
carbohydrates
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 209) Which proteolytic enzyme induces lysis of fibrin during Hbrinolysis?
A)
Fibrin
done
clear
B)
Thrombin
done
clear
C)
Plasmin
done
clear
D)
Platelet factor-VII
done
clear
View Answer play_arrow
question_answer 210) Which of the following enzymes is used to join bits of DNA?
A)
Ligase
done
clear
B)
Primase
done
clear
C)
Endonuclease
done
clear
D)
DNA polymerase
done
clear
View Answer play_arrow
question_answer 211) All eukaryotic genes contain two kinds of base sequences. Which of the following plays role in protein synthesis?
A)
Introns
done
clear
B)
Exons
done
clear
C)
Electrons
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 212) The genetic material of prokaryotic cell is called:
A)
nucleus
done
clear
B)
nucleolus
done
clear
C)
nucleoid
done
clear
D)
centromere
done
clear
View Answer play_arrow
question_answer 213) In prokaryotes, the genetic material is:
A)
linear DNA with histones
done
clear
B)
circular DNA with histones
done
clear
C)
linear DNA without histones
done
clear
D)
circular DNA without histones
done
clear
View Answer play_arrow
question_answer 214) The direction of DNA replication is from:
A)
amino acid end
done
clear
B)
3 end towards 5 end
done
clear
C)
5 end towards 3 end
done
clear
D)
amino terminus to carboxy terminus
done
clear
View Answer play_arrow
question_answer 215) In operon concept, regulator gene functions as:
A)
represser
done
clear
B)
regulator
done
clear
C)
inhibitor
done
clear
D)
initiator
done
clear
View Answer play_arrow
question_answer 216) The importance of meiosis lies in:
A)
bringing discontinuous variations
done
clear
B)
addition in the number of chromosomes
done
clear
C)
reduction in the number of chromosomes
done
clear
D)
maintaining the number of chromosomes
done
clear
View Answer play_arrow
question_answer 217) In mitotic cell division, the division of centromere and the division of chromatid occurs between:
A)
anaphase and telophase
done
clear
B)
prophase and metaphase
done
clear
C)
telophase and interphase
done
clear
D)
anaphase and metaphase
done
clear
View Answer play_arrow
question_answer 218) In which stage of the first meiotic division, each chromosome undergoes longitudinal division to give rise to two sister chromarids?
A)
Zygotene
done
clear
B)
Diplotene
done
clear
C)
Diakinesis
done
clear
D)
Pachytene
done
clear
View Answer play_arrow
question_answer 219) Mirabilis jalapa is an example of:
A)
complete dominance
done
clear
B)
supplementary gene
done
clear
C)
incomplete dominance
done
clear
D)
complementary gene
done
clear
View Answer play_arrow
question_answer 220) Which of the following is dominant character according to Mendel?
A)
Dwarf plant and yellow fruit
done
clear
B)
Terminal fruit and wrinkled seed
done
clear
C)
White testa and yellow pericarp
done
clear
D)
Green coloured fruit and rounded seed
done
clear
View Answer play_arrow
question_answer 221) Lack of independent assortment of genes A and B in fruit fly Drosophila is due to:
A)
repulsion
done
clear
B)
linkage
done
clear
C)
crossing-over
done
clear
D)
recombination
done
clear
View Answer play_arrow
question_answer 222) When two mutations are located in the same functional unit or in different functional units then it is confirmed by:
A)
test cross
done
clear
B)
back cross
done
clear
C)
reciprocal cross
done
clear
D)
complementation test
done
clear
View Answer play_arrow
question_answer 223) Prototherians are the connecting links between:
A)
amphibians and aves
done
clear
B)
reptiles and mammals
done
clear
C)
fishes and amphibians
done
clear
D)
reptiles and amphibinas
done
clear
View Answer play_arrow
question_answer 224) The pioneers in the field of organic evolution are:
A)
Karl Landsteiner, Hugo de Vries, Malthus
done
clear
B)
Darwin, Hugo de Vries, Lamarck, Huxley
done
clear
C)
Lamarck, Karl Landsteiner, Malthus, Hugo de Vries
done
clear
D)
Darwin, Lamarck, Karl Landsteiner, Hugo de Vries
done
clear
View Answer play_arrow
question_answer 225) Darwin finches are related to which of the following evidences?
A)
Fossils
done
clear
B)
Embryology
done
clear
C)
Anatomy
done
clear
D)
Geographical distribution
done
clear
View Answer play_arrow
question_answer 226) Allopatric speciation is due to:
A)
geographical separation of population
done
clear
B)
hybridization between closely related species
done
clear
C)
migration of the members of a species from one to other population
done
clear
D)
both and
done
clear
View Answer play_arrow
question_answer 227) Evolutionary convergence is characterized by:
A)
development of characteristics by random mating
done
clear
B)
replacement of common characteristic in different related groups
done
clear
C)
development of dissimilar characteristics in closely related groups
done
clear
D)
development of a common set of characteristics in groups of different ancestry
done
clear
View Answer play_arrow
question_answer 228) How many sub-phyla are available in Tracheata, according to Tippos classification of kingdom Plantae?
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 229) The usage of binomial names, for plant species, was accepted by all after the publication of the works by:
A)
Hooker
done
clear
B)
Linnaeus
done
clear
C)
Bentham
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 230) What is a key stone species?
A)
A rare species that has minimal impact on biomass and on other species in community
done
clear
B)
A dominant species that constitutes a large proportion of biomass, which affects many other species
done
clear
C)
A common species that has plenty of biomass, yet has a fairly low impact on the communitys organization
done
clear
D)
A species which makes up only a small proportion of the total biomass of a community, yet has a huge impact on the communitys organization and survival
done
clear
View Answer play_arrow
question_answer 231) In biotic community, which of the following can be called protective device?
A)
Mimicry
done
clear
B)
Symbiosis
done
clear
C)
Competition
done
clear
D)
Parasitism
done
clear
View Answer play_arrow
question_answer 232) In which of the following population, genetic drift operates?
A)
Island
done
clear
B)
Smaller
done
clear
C)
Larger
done
clear
D)
Continental
done
clear
View Answer play_arrow
question_answer 233) The driving force of an ecosystem is:
A)
producers
done
clear
B)
biomass
done
clear
C)
solar energy
done
clear
D)
grassland
done
clear
View Answer play_arrow
question_answer 234) The correct match of atmospheric gases is:
A)
nitrogen\[-0.03%,\]oxygen\[-78.08%,\] argon\[-\text{ }0.93%\] and \[C{{O}_{2}}-20.95%\]
done
clear
B)
nitrogen\[-78.08%,\] oxygen\[-20.95%,\]argon\[-0.03%\] and \[C{{O}_{2}}-0.03%\]
done
clear
C)
nitrogen\[-0.03%,\] oxygen\[-78.08%,\] argon\[-\text{ }20.95%\] and \[C{{O}_{2}}-0.93%\]
done
clear
D)
nitrogen\[-78.08%,\] oxygen\[-20.95%,\] argon\[-\text{ }0.93%\] and \[C{{O}_{2}}-0.03%\]
done
clear
View Answer play_arrow
question_answer 235) Zooplanktons are:
A)
parasites
done
clear
B)
primary producers
done
clear
C)
primary consumers
done
clear
D)
primary decomposers
done
clear
View Answer play_arrow
question_answer 236) Photochemical smog formed in congested metropolitan cities mainly consists of:
A)
hydrocarbons, ozone and \[S{{O}_{X}}\]
done
clear
B)
hydrocarbons, \[S{{O}_{2}}\]and \[C{{O}_{2}}\]
done
clear
C)
smoke, peroxyacetyl nitrate and \[S{{O}_{2}}\]
done
clear
D)
ozone, peroxyacetyl nitrate and \[N{{O}_{X}}\]
done
clear
View Answer play_arrow
question_answer 237) Acid rain is due to increase in atmospheric concentration of:
A)
Ozone
done
clear
B)
\[C{{O}_{2}}\]and CO
done
clear
C)
\[S{{O}_{3}}\]and CO
done
clear
D)
\[S{{O}_{2}}\]and nitrogen oxide
done
clear
View Answer play_arrow
question_answer 238) The true statement about green-house effect is that it is caused by:
A)
\[C{{O}_{2}}\]only
done
clear
B)
\[S{{O}_{2}}\] only
done
clear
C)
\[C{{O}_{2}}\]and \[S{{O}_{2}}\]
done
clear
D)
\[C{{O}_{2}},\text{ }CFC,\text{ }C{{H}_{4}}\]and \[N{{O}_{2}}\] gases
done
clear
View Answer play_arrow
question_answer 239) Which of the following statement about viruses is correct?
A)
Viruses are obligate parasites
done
clear
B)
Viruses contain both RNA and DNA
done
clear
C)
Nucleic acid of viruses is known as capsid
done
clear
D)
Viruses possess their own metabolic system
done
clear
View Answer play_arrow
question_answer 240) The virus, that infects bacteria, are made up of:
A)
Protein only
done
clear
B)
RNA and protein
done
clear
C)
DNA and lipid
done
clear
D)
DNA and protein
done
clear
View Answer play_arrow
question_answer 241) The first transgenic crop was:
A)
pea
done
clear
B)
flax
done
clear
C)
tobacco
done
clear
D)
cotton
done
clear
View Answer play_arrow
question_answer 242) One of the major difficulties in the biological control of insect pests is the:
A)
practical difficulty of introducing the predator to specific areas
done
clear
B)
method is less effective as compared with the use of insecticides
done
clear
C)
predator does not always survive when transferred to a new environment
done
clear
D)
predator develops a preference to other diets and may itself become a pest
done
clear
View Answer play_arrow
question_answer 243) Casparian strips are present in:
A)
cortex
done
clear
B)
epidermis
done
clear
C)
endodermis
done
clear
D)
hypodermis
done
clear
View Answer play_arrow
question_answer 244) The function of microvilli is:
A)
cellular movement
done
clear
B)
specialized uptake of macro molecules
done
clear
C)
increase in surface area for absorption
done
clear
D)
extensive movement of substances over cell surface
done
clear
View Answer play_arrow
question_answer 245) Chemiosmotic theory of ATP synthesis, in the chloroplast and mitochondria, is based on:
A)
proton gradient
done
clear
B)
membrane potential
done
clear
C)
accumulation of K ions
done
clear
D)
accumulation of Na ions
done
clear
View Answer play_arrow
question_answer 246) The plants respond to photoperiods due to the presence of:
A)
enzymes
done
clear
B)
stomatas
done
clear
C)
phytochromes
done
clear
D)
phytohormones
done
clear
View Answer play_arrow
question_answer 247) Mesophyll cells, which liberate malic acid at night time, are of:
A)
\[{{C}_{4}}-\] plants
done
clear
B)
\[{{C}_{3}}-\]plants
done
clear
C)
\[{{C}_{2}}-\]plants
done
clear
D)
\[{{C}_{1}}\] plants
done
clear
View Answer play_arrow
question_answer 248) Photorespiration in \[{{C}_{3}}-\] plants starts from:
A)
glycine
done
clear
B)
glycerate
done
clear
C)
phosphoglycolate
done
clear
D)
phosphoglycerate
done
clear
View Answer play_arrow
question_answer 249) Anaerobic respiration is also called:
A)
restoration
done
clear
B)
fragmentation
done
clear
C)
multiplication
done
clear
D)
fermentation
done
clear
View Answer play_arrow
question_answer 250) Biological oxidation in Krebs cycle involves:
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 251) The name of process of aerobic respiration, in which energy is provided in steps in electron transport chain, is:
A)
EMP - pathway
done
clear
B)
decarboxylation
done
clear
C)
photophosphorylation
done
clear
D)
oxidative phosphoryladon
done
clear
View Answer play_arrow
question_answer 252) Asthma is caused due to:
A)
infection of lungs
done
clear
B)
infection of trachea
done
clear
C)
bleeding into pleural cavity
done
clear
D)
spasm in bronchial muscles
done
clear
View Answer play_arrow
question_answer 253) A person breathing normally -at rest, takes in and expels approximately half a litre of air during each respiratory cycle. This is called:
A)
tidal volume
done
clear
B)
vital capacity
done
clear
C)
inspiratory reserve volume
done
clear
D)
expiratory reserve volume
done
clear
View Answer play_arrow
question_answer 254) The Largest quantity of air that can be expired, after maximal inspiration, is called:
A)
tidal volume
done
clear
B)
vital capacity
done
clear
C)
residual volume
done
clear
D)
total lung volume
done
clear
View Answer play_arrow
question_answer 255) Rate of heart beat is determined by:
A)
AV - node
done
clear
B)
SA - node
done
clear
C)
Purkinje fibres
done
clear
D)
Papillary muscles
done
clear
View Answer play_arrow
question_answer 256) The first heart sound is produced when:
A)
diastole begins
done
clear
B)
semilunar valve close quickly
done
clear
C)
interventricular pressure decreases
done
clear
D)
bicuspid and tricuspid valve close quickly
done
clear
View Answer play_arrow
question_answer 257) Which of the following layer of heart wall consists of cardiac muscles?
A)
Endocardium
done
clear
B)
Myocardium
done
clear
C)
Epicardium
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 258) If heart beats 75 beats/ min. then what is time for cardiac cycle?
A)
0.5 sec
done
clear
B)
0.8 sec
done
clear
C)
1 sec
done
clear
D)
1.5 sec
done
clear
View Answer play_arrow
question_answer 259) Blood pressure increases and heart rate decreases in response to:
A)
exercise
done
clear
B)
haemorrhage
done
clear
C)
exposure to high altitude
done
clear
D)
increased intracranial pressure
done
clear
View Answer play_arrow
question_answer 260) P wave of ECG occurs before the:
A)
onset of ventricular ejection
done
clear
B)
end of atrial contraction
done
clear
C)
begining of atrial contraction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 261) Liver in our body stores:
A)
vitamin - A
done
clear
B)
vitamin - D
done
clear
C)
vitamin \[-\text{ }{{B}_{12}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 262) Secrerin hormone is secreted by:
A)
liver
done
clear
B)
pancreas
done
clear
C)
intestine
done
clear
D)
Brunners glands
done
clear
View Answer play_arrow
question_answer 263) The contraction of gall bladder is due to:
A)
gastrin
done
clear
B)
secretin
done
clear
C)
enterogasterone
done
clear
D)
cholecystokinin
done
clear
View Answer play_arrow
question_answer 264) Which of the following is the character of the bile juice?
A)
It has trypsin
done
clear
B)
It has no enzyme
done
clear
C)
It has enterogasterone
done
clear
D)
It has tripophnomide
done
clear
View Answer play_arrow
question_answer 265) Average pH of human urine is:
A)
6.0
done
clear
B)
9.0
done
clear
C)
3.0
done
clear
D)
7.0
done
clear
View Answer play_arrow
question_answer 266) Cells present in the inner lining of kidneys are:
A)
podocytes
done
clear
B)
choanocytes
done
clear
C)
pinocytes
done
clear
D)
nephrocytes
done
clear
View Answer play_arrow
question_answer 267) Which of the following is impermeable to water?
A)
Vertical limb of loop of Henie
done
clear
B)
Descending limb of loop of Henie
done
clear
C)
Ascending limb of loop of Henie
done
clear
D)
Both a and b
done
clear
View Answer play_arrow
question_answer 268) Ducts of Bellini are present in:
A)
liver
done
clear
B)
kidney
done
clear
C)
intestine
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 269) Human brain has greater development of:
A)
cerebrum
done
clear
B)
cerebellum
done
clear
C)
optic lobes
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 270) The end organs of Ruffini are receptors of:
A)
heat
done
clear
B)
cold
done
clear
C)
pressure
done
clear
D)
touch
done
clear
View Answer play_arrow
question_answer 271) Which of the following part of human brain is associated with integration of sympathetic and parasympathetic activities?
A)
Cerebrum
done
clear
B)
Neopallium
done
clear
C)
Hypothalamus
done
clear
D)
Medulla oblongata
done
clear
View Answer play_arrow
question_answer 272) The unidirectional transmission of a nerve impulse through nerve fibre is due to:
A)
neurotransmitters are released by axon endings
done
clear
B)
neurotransmitters which are released by dendrites
done
clear
C)
nerve fibre which is insulated by a medullary sheath
done
clear
D)
sodium pump which starts operating into the nerve fibre
done
clear
View Answer play_arrow
question_answer 273) In the myopia eye defect, the rays of light:
A)
do not enter the eye at all
done
clear
B)
meet at a focus in front of the retina
done
clear
C)
come to a focus at back of retina
done
clear
D)
come to a focus in between retina and iris
done
clear
View Answer play_arrow
question_answer 274) Sensory receptor of warmth located principally at the tip of fingers is known as:
A)
Webers organ
done
clear
B)
organ of Giraldes
done
clear
C)
Ruffinis corpuscles
done
clear
D)
organ of Zuckerkandl
done
clear
View Answer play_arrow
question_answer 275) Hormones secreted by pancreas are:
A)
ACTH
done
clear
B)
oxytocin
done
clear
C)
LH and FSH
done
clear
D)
insulin and glucagon
done
clear
View Answer play_arrow
question_answer 276) Neurohypophysis secretes:
A)
ADH and oxytocin
done
clear
B)
oxytocin and estrogen
done
clear
C)
vasopressin and GH
done
clear
D)
vasopressin and estrogen
done
clear
View Answer play_arrow
question_answer 277) Secretion of androgens by testes is regulated by:
A)
LTH
done
clear
B)
FSH
done
clear
C)
ICSH
done
clear
D)
Oxytocin
done
clear
View Answer play_arrow
question_answer 278) Pancreatic duct of a healthy dog is blocked. Which of the functions of pancreas will not be affected?
A)
Protein digestion
done
clear
B)
Carbohydrate digestion
done
clear
C)
Neutralization of chyme
done
clear
D)
Maintenance of normal blood sugar level
done
clear
View Answer play_arrow
question_answer 279) Physiologically active thyroxine exists in which of the following form?
A)
Unbound
done
clear
B)
Bound to albumin
done
clear
C)
Bound to globulin
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 280) A flower characterized by monadelphous tubular stamens belongs to:
A)
Solanaceae
done
clear
B)
Liliaceae
done
clear
C)
Malvaceae
done
clear
D)
Brassicaceae
done
clear
View Answer play_arrow
question_answer 281) In Musa, inflorescence is:
A)
spadix
done
clear
B)
corymb
done
clear
C)
capitulum
done
clear
D)
polychasial cyme
done
clear
View Answer play_arrow
question_answer 282) The formation of gametophyte, from sporophyte, without spore formation or without meiosis is known as:
A)
apospory
done
clear
B)
apogamy
done
clear
C)
parthenogenesis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 283) Anemophillous flowers have:
A)
sessile stigma
done
clear
B)
small and smooth stigma
done
clear
C)
colored flower
done
clear
D)
large and feathery stigma
done
clear
View Answer play_arrow
question_answer 284) In oogamy, fertilization involves:
A)
a small non-motile female gamete and a large motile male gamete
done
clear
B)
a large motile female gamete and a small non-motile male gamete
done
clear
C)
a large non-motile female gamete and a small motile male gamete
done
clear
D)
a large non-motile female gamete and a small non-motile male gamete
done
clear
View Answer play_arrow
question_answer 285) In angiosperms, triple fusion results in the formation of:
A)
zygotic nucleus
done
clear
B)
polar nucleus
done
clear
C)
secondary nucleus
done
clear
D)
primary endosperm nucleus
done
clear
View Answer play_arrow
question_answer 286) During a womans life time, she produces about:
A)
40-50 eggs
done
clear
B)
300-350 eggs
done
clear
C)
400-500 eggs
done
clear
D)
750-850 eggs
done
clear
View Answer play_arrow
question_answer 287) The production and maturation of sperm in testes is known as:
A)
oogenesis
done
clear
B)
sporogenesis
done
clear
C)
gametogenesis
done
clear
D)
spermatogenesis
done
clear
View Answer play_arrow
question_answer 288) The phase of menstrual cycle in humans that lasts for 7-8 days, is:
A)
menstruation
done
clear
B)
luteal phase
done
clear
C)
ovulatory phase
done
clear
D)
follicular phase
done
clear
View Answer play_arrow
question_answer 289) Which one of the following statement with regard to the embryonic development in humans is correct?
A)
Cleavage results in a hollow balls of cells called morula
done
clear
B)
Cleavage in mammalian ova is unequal holoblastic and horizontal
done
clear
C)
Rearrangement of blastomeres, a central cavity is formed inside the morula
done
clear
D)
Cleavage bring about considerable increase in the mass of protoplasm
done
clear
View Answer play_arrow
question_answer 290) The most accepted theory of ageing is:
A)
less RBC in blood
done
clear
B)
thymus gland becomes non - functional
done
clear
C)
brain cells die with ageing
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 291) Which of the following is not immunized by triple antigen?
A)
Typhoid
done
clear
B)
Tetanus
done
clear
C)
Diptheria
done
clear
D)
Whooping cough
done
clear
View Answer play_arrow
question_answer 292) A person with the sex chromosomes XXY suffers from:
A)
Downs syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Gynandromorphism
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 293) Which of the following represents Klinefelters syndrome?
A)
XX
done
clear
B)
XO
done
clear
C)
XY
done
clear
D)
XXY
done
clear
View Answer play_arrow
question_answer 294) Which is the closest pet of human being?
A)
Cat
done
clear
B)
Cow
done
clear
C)
Dog
done
clear
D)
Buffalo
done
clear
View Answer play_arrow
question_answer 295) Th bacterial disease which is found in chickens, is:
A)
rickets
done
clear
B)
Ranikhets
done
clear
C)
fowl fox
done
clear
D)
fowl cholera
done
clear
View Answer play_arrow
question_answer 296) Which of the following is a viral disease in silkworm?
A)
Flacherie
done
clear
B)
Grasserie
done
clear
C)
Muscardine
done
clear
D)
Pebrine
done
clear
View Answer play_arrow
question_answer 297) Which of the following is not the example of marine fishes?
A)
Labeo
done
clear
B)
mugil
done
clear
C)
Hilsa
done
clear
D)
Sardines
done
clear
View Answer play_arrow
question_answer 298) Reproducing new plants by cells, instead of seeds, is known as:
A)
Mutation
done
clear
B)
antibiotics
done
clear
C)
biofertilizer
done
clear
D)
tissue culture
done
clear
View Answer play_arrow
question_answer 299) Creosote is used to prevent:
A)
rusts of wheat
done
clear
B)
dry rot of wood
done
clear
C)
loose smut of oats
done
clear
D)
brown rust of barley
done
clear
View Answer play_arrow
question_answer 300) Which of the following insecticide is obtained from the roots of Denis elliptica?
A)
Cinerin
done
clear
B)
Nicotine
done
clear
C)
Rotenone
done
clear
D)
Pyrethrum
done
clear
View Answer play_arrow