question_answer 1) Which activity is not based upon friction?
A)
Writing
done
clear
B)
Speaking
done
clear
C)
Hearing
done
clear
D)
Walking
done
clear
View Answer play_arrow
question_answer 2) The maximum time period of any simple pendulum on the earth is
A)
180.5 min
done
clear
B)
100 min
done
clear
C)
90.5 min
done
clear
D)
84.5 min
done
clear
View Answer play_arrow
question_answer 3) The mathematical equation for magnetic field lines of force is
A)
\[\vec{\nabla }.\mathbf{\vec{B}}=0\]
done
clear
B)
\[\vec{\nabla }.\mathbf{\vec{B}}\ne 0\]
done
clear
C)
\[\vec{\nabla }.\mathbf{\vec{B}}>0\]
done
clear
D)
\[\vec{\nabla }.\mathbf{\vec{B}}<0\]
done
clear
View Answer play_arrow
question_answer 4) The distance at which the magnetic field on axis as compared to the magnetic field at the centre of the coil carrying current I and radius R is \[\frac{1}{8}\]would be
A)
R
done
clear
B)
\[\sqrt{2}R\]
done
clear
C)
2R
done
clear
D)
\[\sqrt{3}R\]
done
clear
View Answer play_arrow
question_answer 5) The earth has volume V and surface area A, then capacitance would be
A)
\[4\pi {{\varepsilon }_{0}}\frac{A}{V}\]
done
clear
B)
\[4\pi {{\varepsilon }_{0}}\frac{V}{A}\]
done
clear
C)
\[12\pi {{\varepsilon }_{0}}\frac{V}{A}\]
done
clear
D)
\[12\pi {{\varepsilon }_{0}}\frac{A}{V}\]
done
clear
View Answer play_arrow
question_answer 6) Capacitors are used in electrical circuits where appliances need more
A)
current
done
clear
B)
voltage
done
clear
C)
watt
done
clear
D)
resistance
done
clear
View Answer play_arrow
question_answer 7) If the circumference of a sphere is 2 m, then capacitance of sphere in water would be
A)
2700 pF
done
clear
B)
2760pF
done
clear
C)
2780 pF
done
clear
D)
2800 pF
done
clear
View Answer play_arrow
question_answer 8) An electric pump is used to fill an overhead tank of capacity 9 m3 kept at a height of 10 m above the ground. If the pump takes 5 min to fill the tank by consuming 10 kW power the efficiency of the pump should be
A)
60%
done
clear
B)
40%
done
clear
C)
20%
done
clear
D)
30%
done
clear
View Answer play_arrow
question_answer 9) If we consider solar system consisting of the earth and sun only as of the ideal thermodynamic system. The sun works as source of energy having temperature 6000 K and earth as sink having temperature 300 K, the efficiency of solar system would be on the basis of exchange of radiations
A)
30%
done
clear
B)
65%
done
clear
C)
75%
done
clear
D)
95%
done
clear
View Answer play_arrow
question_answer 10) Which is the most elastic?
A)
Iron
done
clear
B)
Copper
done
clear
C)
Quartz
done
clear
D)
Wood
done
clear
View Answer play_arrow
question_answer 11) \[Y=\frac{mgl}{\pi {{r}^{2}}L}\] formula would give Y, if mg is doubled
A)
2Y
done
clear
B)
\[\frac{Y}{2}\]
done
clear
C)
Y
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 12) Ratio between maximum range and square of time of flight in projectile motion is
A)
10 : 49
done
clear
B)
49 : 10
done
clear
C)
98 : 10
done
clear
D)
10 : 98
done
clear
View Answer play_arrow
question_answer 13) A motor cycle racer takes a round with speed \[20\,m{{s}^{-1}}\] in a curvature of radius of R = 40 m, then the leaning angle of motor cycle for safe turn is \[(g=10\,m{{s}^{-2}})\])
A)
\[20{}^\circ \]
done
clear
B)
\[30{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[60{}^\circ \]
done
clear
View Answer play_arrow
question_answer 14) Gauss law of gravitation is
A)
\[\oint{\mathbf{\vec{g}}.d\vec{s}=m}\]
done
clear
B)
\[\oint{\mathbf{\vec{g}}.d\vec{s}=Gm}\]
done
clear
C)
\[\oint{\mathbf{\vec{g}}.d\vec{s}=4G\pi m}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 15) Photoelectric effect is an example of
A)
elastic collision
done
clear
B)
inelastic collision
done
clear
C)
two dimensional collision
done
clear
D)
oblique collision
done
clear
View Answer play_arrow
question_answer 16) The molar heat capacity in a process of a diatomic gas, if it does a work of \[\frac{Q}{4}\] when a heat of Q is supplied to it is
A)
\[\frac{2R}{5}\]
done
clear
B)
\[\frac{5R}{2}\]
done
clear
C)
\[\frac{10R}{3}\]
done
clear
D)
\[\frac{6R}{7}\]
done
clear
View Answer play_arrow
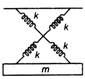
question_answer 17)
As shown in figure, a simple harmonic motion oscillator having identical four springs has time period
A)
\[T=2\pi \sqrt{\frac{m}{4k}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{m}{2k}}\]
done
clear
C)
\[T=2\pi \sqrt{\frac{m}{k}}\]
done
clear
D)
\[T=2\pi \sqrt{\frac{2m}{k}}\]
done
clear
View Answer play_arrow
question_answer 18)
A)
c + v
done
clear
B)
c - v
done
clear
C)
c x v
done
clear
D)
c
done
clear
View Answer play_arrow
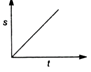
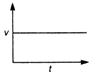
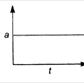
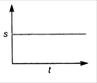
question_answer 19) Which graph represents a state of rest for an object?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
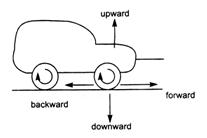
question_answer 20)
Direction of frictional force between wheel of the car and road is
A)
upward
done
clear
B)
forward
done
clear
C)
backward
done
clear
D)
downward
done
clear
View Answer play_arrow
question_answer 21) For different capillaries of radius r, the condition of liquid rise (h) to surface tension is
A)
\[rh=\] constant
done
clear
B)
\[\frac{h}{r}=cons\tan t\]
done
clear
C)
h + r = constant
done
clear
D)
h - r = constant
done
clear
View Answer play_arrow
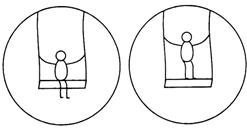
question_answer 22)
A child swings sitting and standing inside swing as shown in figure, then period of oscillations have the relation
A)
\[{{(T)}_{sitting}}={{(T)}_{S\tan ding}}\]
done
clear
B)
\[{{(T)}_{sitting}}>{{(T)}_{S\tan ding}}\]
done
clear
C)
\[{{(T)}_{sitting}}<{{(T)}_{S\tan ding}}\]
done
clear
D)
\[2{{(T)}_{sitting}}={{(T)}_{S\tan ding}}\]
done
clear
View Answer play_arrow
question_answer 23) The ratio between Bohr radii are
A)
1 : 2 : 3
done
clear
B)
2 : 4 : 6
done
clear
C)
1 : 4 : 9
done
clear
D)
1 : 3 : 5
done
clear
View Answer play_arrow
question_answer 24) If half-life of a radio isotope is 2 s and number of atoms are only 4, then after one half-life remaining (without decay) atoms are probably
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 25) \[\frac{h}{2\pi }\]is the dimension of
A)
velocity
done
clear
B)
momentum
done
clear
C)
energy
done
clear
D)
angular momentum
done
clear
View Answer play_arrow
question_answer 26) Which of the following has high pitch in their sound?
A)
Lion
done
clear
B)
Mosquito
done
clear
C)
Man
done
clear
D)
Woman
done
clear
View Answer play_arrow
question_answer 27) Geostationary satellite
A)
falls with g towards the earth
done
clear
B)
has period of 24h
done
clear
C)
has equatorial orbit
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 28) The potential on the hollow sphere of radius1 m is 1000 V, then potential at \[\frac{1}{4}\] m from the centre of the sphere is
A)
1000 V
done
clear
B)
500 V
done
clear
C)
250 V
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 29) Electric field (E) and current density (J) have relation
A)
\[E\propto {{J}^{-1}}\]
done
clear
B)
\[E\propto J\]
done
clear
C)
\[E\propto \frac{1}{{{J}^{2}}}\]
done
clear
D)
\[{{E}^{2}}\propto \frac{1}{J}\]
done
clear
View Answer play_arrow
question_answer 30) Time constant of LC circuit is
A)
\[\frac{1}{2\pi LC}\]
done
clear
B)
\[\frac{1}{2\pi {{L}^{2}}{{C}^{2}}}\]
done
clear
C)
\[\frac{LC}{2\pi }\]
done
clear
D)
\[2\pi \sqrt{LC}\]
done
clear
View Answer play_arrow
question_answer 31) The value of Planck energy is
A)
\[\frac{nhc}{\lambda }\]
done
clear
B)
\[nh\lambda \]
done
clear
C)
\[nhc\lambda \]
done
clear
D)
\[\frac{nh\lambda }{c}\]
done
clear
View Answer play_arrow
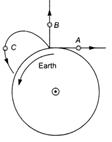
question_answer 32)
A body A moves with constant velocity on a straight line path tangential to the earths surface. Another body B is thrown vertically upwards, it goes to a height and falls back on earth. A third body C is projected to an angle and follows a parabolic path as shown in figure. The bodies whose angular momentum relative to the centre of the earth is conserved are
A)
B only
done
clear
B)
B and C
done
clear
C)
A, B, C
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) Which unit is not for length?
A)
Parsec
done
clear
B)
Light year
done
clear
C)
Angstrom
done
clear
D)
Nano
done
clear
View Answer play_arrow
question_answer 34) Which one is angular resolution fundamental quantity?
A)
Length
done
clear
B)
Time
done
clear
C)
Radian
done
clear
D)
Angle
done
clear
View Answer play_arrow
question_answer 35) Three masses of 2 kg, 4 kg and 4 kg are placed at the three points (1, 0, 0), (1, 1, 0) and (0, 1, 0) respectively. The position vector of its centre of mass is
A)
\[\frac{3}{5}\mathbf{\hat{i}}+\frac{4}{5}\mathbf{\hat{j}}\]
done
clear
B)
\[(3\mathbf{\hat{i}}+\mathbf{\hat{j}})\]
done
clear
C)
\[\frac{2}{5}\mathbf{\hat{i}}+\frac{4}{5}\mathbf{\hat{j}}\]
done
clear
D)
\[\frac{1}{5}\mathbf{\hat{i}}+\frac{3}{5}\mathbf{\hat{j}}\]
done
clear
View Answer play_arrow
question_answer 36) Let A = light obtained by stimulated emission and B = light obtained by spontaneous emission, then
A)
A is incoherent, B is incoherent
done
clear
B)
A is incoherent, B is coherent
done
clear
C)
A is coherent, B is coherent
done
clear
D)
A is coherent, B is incoherent
done
clear
View Answer play_arrow
question_answer 37)
Irreducible area abed, in figure is
A)
work
done
clear
B)
planck constant
done
clear
C)
joule
done
clear
D)
charge
done
clear
View Answer play_arrow
question_answer 38) For which of the following processes is the entropy change zero?
A)
Isobaric
done
clear
B)
Isothermal
done
clear
C)
Adiabatic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 39) If absolute zero is \[-273.15\text{ }{}^\circ C\] on Celsius temperature scale, then the absolute zero on the Fahrenheit scale is
A)
\[-273.15\text{ }{}^\circ F\]
done
clear
B)
\[-\text{ }453.15\text{ }{}^\circ F\]
done
clear
C)
\[-\text{ }459.67\text{ }{}^\circ F\]
done
clear
D)
\[-\text{ }491.67\text{ }{}^\circ F\]
done
clear
View Answer play_arrow
question_answer 40) The average binding energy per nucleon is maximum for the nucleus
A)
\[_{2}H{{e}^{4}}\]
done
clear
B)
\[_{8}{{O}^{16}}\]
done
clear
C)
\[_{26}F{{e}^{56}}\]
done
clear
D)
\[_{92}{{U}^{238}}\]
done
clear
View Answer play_arrow
question_answer 41) If an\[\alpha \]-particle of mass m, charge q and velocity v is incident on a nucleus of charge Q and mass m, then the distance of closest approach is
A)
\[\frac{Qq}{4\pi {{\varepsilon }_{0}}{{m}^{2}}{{v}^{2}}}\]
done
clear
B)
\[\frac{Qq}{2\pi {{\varepsilon }_{0}}m{{v}^{2}}}\]
done
clear
C)
\[\frac{Qqm{{v}^{2}}}{2}\]
done
clear
D)
\[\frac{Qq}{m{{v}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 42) The de-Broglie wavelength of a particle with mass m and kinetic energy K is
A)
\[\frac{h}{\sqrt{2mk}}\]
done
clear
B)
\[\frac{h}{k}\]
done
clear
C)
\[\frac{hk}{2mc}\]
done
clear
D)
\[\frac{hc}{2mk}\]
done
clear
View Answer play_arrow
question_answer 43) Momentum of photon is
A)
\[\frac{hv}{{{c}^{2}}}\]
done
clear
B)
\[\frac{hv}{{{c}^{3}}}\]
done
clear
C)
\[\frac{hv}{c}\]
done
clear
D)
\[\frac{h}{c}\]
done
clear
View Answer play_arrow
question_answer 44) Following process is known as \[hv\to {{e}^{+}}+{{e}^{-}}\]
A)
Pair production
done
clear
B)
Photoelectric effect
done
clear
C)
Compton effect
done
clear
D)
Zeeman effect
done
clear
View Answer play_arrow
question_answer 45) Choose the correct statement.
A)
Photoelectric effect can take place from bound electron
done
clear
B)
Photoelectric effect can take place from free electron
done
clear
C)
Photoelectric effect can take place from bounded or free electron
done
clear
D)
Nothing can be said
done
clear
View Answer play_arrow
question_answer 46) Assertion The formula connecting u, v and \[f\] for a spherical mirror is valid only for mirrors radii of curvature. Reason Laws of reflection are strictly valid for plane surfaces, but not for large spherical surface.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 47) Assertion Endoscopy in vovles use of optical fibres to study internal organs. Reason Optical fibres are based on phenomenon of total internal reflection.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 48) Assertion A ship floats higher in the water on a high pressure day than on a low pressure day. Reason Floating of ship in the water is not possible because of buoyancy force which is present due to pressure difference.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 49) Assertion A proton and an alpha particle having the same kinetic energy are moving in circular paths in a uniform magnetic field. The radii of their circular paths will be equal. Reason Any two charged particles having equal kinetic energies and entering a region of uniform magnetic field \[\mathbf{\vec{B}}\] in a direction perpendicular to \[\mathbf{\vec{B}}\], will describe circular trajectories of equal radii.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 50) Assertion The clouds in sky generally appear to be whitish. Reason Diffraction due to clouds is efficient in equal measure at all wavelengths.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 51) For a reaction \[2S{{O}_{2}}(s)+{{O}_{2}}(g)\rightleftharpoons 2S{{O}_{3}}(g);\]\[\Delta H=-188.3\,kJ.\] The number of moles of \[S{{O}_{3}}\] formed is increased if
A)
temperature is increased at constant volume
done
clear
B)
Inert gas is added to the mixture
done
clear
C)
\[{{\text{O}}_{\text{2}}}\]is removed from the mixture
done
clear
D)
volume of the reaction flask is decreased
done
clear
View Answer play_arrow
question_answer 52) For a first order reaction \[\text{A}\xrightarrow{{}}\] Products, the half-life is 100 s. The rate constant of the reaction is
A)
\[6.9\times {{10}^{-2}}{{s}^{-1}}\]
done
clear
B)
\[6.93\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
C)
\[6.93\times {{10}^{-3\text{ }}}{{s}^{-1}}\]
done
clear
D)
\[6.93\times {{10}^{-1\text{ }}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 53) The rate of a gaseous reaction triples when temperature is increased by \[\text{1}{{\text{0}}^{\text{o}}}\text{C}\] from \[\text{2}{{\text{5}}^{\text{o}}}\text{C}\text{.}\]The energy of activation of the reaction(in \[\text{kJ}\,\text{mo}{{\text{l}}^{-1}}\]) will be
A)
40
done
clear
B)
70
done
clear
C)
83.8
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 54) The substance which does not show sharp melting point is
A)
\[KCl\]
done
clear
B)
glass
done
clear
C)
ice
done
clear
D)
diamond
done
clear
View Answer play_arrow
question_answer 55) The total number of Bravais lattice in a crystal is
A)
7
done
clear
B)
14
done
clear
C)
230
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 56) Two moles of an ideal gas expand spontaneously into vacuum. The work done is
A)
2 J
done
clear
B)
4 J
done
clear
C)
zero
done
clear
D)
infinity
done
clear
View Answer play_arrow
question_answer 57) The heat change at constant volume \[({{Q}_{v}})\] is equal to
A)
\[\Delta E\]
done
clear
B)
\[\Delta H\]
done
clear
C)
RT
done
clear
D)
\[\Delta G\]
done
clear
View Answer play_arrow
question_answer 58) For a gaseous reaction at 300 K. \[\Delta H=\Delta E=-4.98\,kJ,\] assuming that\[R=8.3\,J{{K}^{-1}}\,mo{{l}^{-1}},\,\Delta {{n}_{(g)}}\]is
A)
1
done
clear
B)
2
done
clear
C)
\[-2\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 59) The pH of a solution is 4. Its \[[{{H}^{+}}]\] is
A)
4 M
done
clear
B)
\[{{10}^{4}}\,M\]
done
clear
C)
\[{{10}^{-4}}\,M\]
done
clear
D)
\[{{10}^{-1}}\,M\]
done
clear
View Answer play_arrow
question_answer 60) The bonds present in \[{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]are
A)
only convalent
done
clear
B)
only ionic
done
clear
C)
covalent and coordinate
done
clear
D)
covalent and ionic
done
clear
View Answer play_arrow
question_answer 61) The isotonic solutions have
A)
the same freezing point
done
clear
B)
the same boiling point
done
clear
C)
the same surface tension
done
clear
D)
the same osmotic pressure
done
clear
View Answer play_arrow
question_answer 62) The radioactive series whose end product is\[_{\text{89}}^{\text{209}}\text{Bi}\]is
A)
Thorium series
done
clear
B)
Fourier series
done
clear
C)
Actinium series
done
clear
D)
Neptunium series
done
clear
View Answer play_arrow
question_answer 63) \[_{6}^{13}C\]and \[_{\text{7}}^{\text{14}}\text{N}\] are the
A)
isotopes
done
clear
B)
isotones
done
clear
C)
isobars
done
clear
D)
isosters
done
clear
View Answer play_arrow
question_answer 64) Electron affinity is positive when
A)
\[{{\text{O}}^{-}}\]is formed from O
done
clear
B)
\[{{\text{O}}^{2-}}\]is formed from \[{{\text{O}}^{-}}\]
done
clear
C)
\[{{\text{O}}^{+}}\]is formed from O
done
clear
D)
\[{{\text{O}}^{3-}}\]is formed from \[{{\text{O}}^{-}}\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following set has all the colored ions?
A)
\[C{{u}^{+}},\,C{{u}^{2+}},\,N{{i}^{2+}}\]
done
clear
B)
\[C{{u}^{2+}},C{{O}^{2+}},S{{c}^{3+}}\]
done
clear
C)
\[C{{u}^{2+}},F{{e}^{2+}},C{{O}^{2+}}\]
done
clear
D)
\[N{{a}^{+}},M{{g}^{2+}},A{{l}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following ions has the smallest radius?
A)
\[T{{i}^{2+}}\]
done
clear
B)
\[N{{i}^{2+}}\]
done
clear
C)
\[P{{t}^{2+}}\]
done
clear
D)
\[Z{{r}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 67) Maximum oxidation state is shown by
A)
\[Os\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Co\]
done
clear
D)
\[Cr\]
done
clear
View Answer play_arrow
question_answer 68) Maximum magnetic moment is shown by
A)
\[{{d}^{5}}\]
done
clear
B)
\[{{d}^{6}}\]
done
clear
C)
\[{{d}^{7}}\]
done
clear
D)
\[{{d}^{8}}\]
done
clear
View Answer play_arrow
question_answer 69) Iron obtained from blast furnace is
A)
wrought iron
done
clear
B)
cast iron
done
clear
C)
pig iron
done
clear
D)
steel
done
clear
View Answer play_arrow
question_answer 70) Which of the following does not contain Mg?
A)
Magnetite
done
clear
B)
Absestos
done
clear
C)
Magnesite
done
clear
D)
Camallite
done
clear
View Answer play_arrow
question_answer 71) Carborundum is
A)
\[Ca{{C}_{2}}\]
done
clear
B)
\[~CaC{{O}_{3}}\]
done
clear
C)
\[F{{e}_{3}}C\]
done
clear
D)
\[SiC\]
done
clear
View Answer play_arrow
question_answer 72) Inorganic benzene is
A)
\[{{B}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{B}_{3}}{{N}_{3}}{{H}_{6}}\]
done
clear
C)
\[{{B}_{3}}{{O}_{3}}{{H}_{6}}\]
done
clear
D)
\[{{(B{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) The molar concentration of 32 g of oxygen in 10 L is
A)
\[3.2\,mol\,{{L}^{-1}}\]
done
clear
B)
\[3.2\,\,g\,{{L}^{-1}}\]
done
clear
C)
\[1.00\,mol\,{{L}^{-1}}\]
done
clear
D)
\[0.10\,mol\,{{L}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 74) The laughing gas is
A)
nitrous oxide
done
clear
B)
nitrogen trioxide
done
clear
C)
nitric oxide
done
clear
D)
nitrogen penta oxide
done
clear
View Answer play_arrow
question_answer 75) Which of the following compound has highest boiling point?
A)
n-hexane
done
clear
B)
n-pentane
done
clear
C)
2-methyl butane
done
clear
D)
2, 2-dimethyl propane
done
clear
View Answer play_arrow
question_answer 76) The reaction, \[C{{H}_{3}}C{{H}_{2}}Cl\xrightarrow[2-CuI]{1-Li}\xrightarrow{C{{H}_{3}}C{{H}_{2}}Cl}\]\[n-\]butane is known as
A)
Wurtz synthesis
done
clear
B)
Corey-House synthesis
done
clear
C)
Kolbe synthesis
done
clear
D)
Friedel-Crafts synthesis
done
clear
View Answer play_arrow
question_answer 77) The total number of isomers of\[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{10}}}\text{O}\]will be
A)
4
done
clear
B)
5
done
clear
C)
6
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 78)
A)
2, 4 butan-di-one
done
clear
B)
ethanoic anhydride
done
clear
C)
ethoxyethanone
done
clear
D)
acetic anhydride
done
clear
View Answer play_arrow
question_answer 79) Dyes are generally obtained from the substances obtained from
A)
petroleum products
done
clear
B)
coal-tar
done
clear
C)
gasoline
done
clear
D)
water gas
done
clear
View Answer play_arrow
question_answer 80) \[2{{C}_{6}}{{H}_{5}}CHO\xrightarrow{NaOH}{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH+{{C}_{6}}{{H}_{5}}COONa\]The same reaction can take place with which ofthe following aldehydes ?
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}CCHO\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHCHO\]
done
clear
View Answer play_arrow
question_answer 81) Among the following compounds, the strongest acid is
A)
\[CH\equiv CH\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}H{{ }_{6}}\]
done
clear
D)
\[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 82) Which of the following compounds will react with ethanolic KCN?
A)
Ethyl chloride
done
clear
B)
Acetyl chloride
done
clear
C)
Benzaldehyde
done
clear
D)
Chlorobenzene
done
clear
View Answer play_arrow
question_answer 83) Ethylamine can be prepared by the action of bromine and caustic potash on
A)
Acetamide
done
clear
B)
Propionamide
done
clear
C)
Formamide
done
clear
D)
Methyl cyanide
done
clear
View Answer play_arrow
question_answer 84) Tonics in general contain
A)
Ether
done
clear
B)
Methanol
done
clear
C)
Ethanol
done
clear
D)
Rectified spirit
done
clear
View Answer play_arrow
question_answer 85) Acetone will be obtained by the ozonolysis of
A)
1-butene
done
clear
B)
2-butene
done
clear
C)
iso-butene
done
clear
D)
2-butyne
done
clear
View Answer play_arrow
question_answer 86) Fog is an example of colloidal system of
A)
liquid dispersed in a liquid
done
clear
B)
liquid dispersed in a gas
done
clear
C)
gas dispersed in a liquid
done
clear
D)
solid dispersed in a solid
done
clear
View Answer play_arrow
question_answer 87) An emulsion is a colloidal solution of one of the following dispersed in another liquid
A)
solid
done
clear
B)
liquid
done
clear
C)
gas
done
clear
D)
medium
done
clear
View Answer play_arrow
question_answer 88) One Faraday is equal to
A)
96.500 C
done
clear
B)
9650 C
done
clear
C)
965.0 C
done
clear
D)
96500 C
done
clear
View Answer play_arrow
question_answer 89) What is\[{{\text{G}}^{\text{o}}}\]at 298 K for the reaction,\[\text{2VO}_{2}^{+}(aq)+4{{H}^{+}}(aq)+Cd(s)\xrightarrow{{}}2V{{O}^{2+}}(aq)\] \[+2{{H}_{2}}O(l)+C{{d}^{2+}}(aq)\]
A)
\[-271\,kJ\]
done
clear
B)
\[1.403\,J\]
done
clear
C)
\[-\text{ }135\text{ }kJ\]
done
clear
D)
\[-\text{ }115\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following shows a metal being oxidized?
A)
\[2Na+2{{H}_{2}}O\xrightarrow{{}}2NaOH+{{H}_{2}}\]
done
clear
B)
\[Cu\xrightarrow{{}}C{{u}^{2+}}+2{{e}^{-}}\]
done
clear
C)
\[C{{u}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Cu\]
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 91) The site of oxidation in an electrochemical cell is
A)
the anode
done
clear
B)
the cathode
done
clear
C)
the electrode
done
clear
D)
reference electrode
done
clear
View Answer play_arrow
question_answer 92) Which metal is used as a coating on steel to prevent corrosion?
A)
Na
done
clear
B)
Ca
done
clear
C)
K
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 93) Hydrolysis of the salt of a strong acid and weak base will
A)
increase with increase in temperature
done
clear
B)
decrease with increase in temperature
done
clear
C)
remains unaffected with change in temperature
done
clear
D)
remain sun effected with change in concentration of the salt
done
clear
View Answer play_arrow
question_answer 94) Dissociation constant of a weak acid is decreased by
A)
addition of a strong acid
done
clear
B)
addition of a salt of the above weak acid
done
clear
C)
decreasing temperature
done
clear
D)
dilution of the solution
done
clear
View Answer play_arrow
question_answer 95) \[\frac{e}{m}\]ratio was determined by
A)
\[\text{J}\,\text{J}\] Thomson
done
clear
B)
Dalton
done
clear
C)
Chadwick
done
clear
D)
Goldstein
done
clear
View Answer play_arrow
question_answer 96) Assertion Sodium-2-dodecyl benzenesulphon ate is a biodegradable detergent. Reason Detergents having highly branched chains are biodegradable.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 97) Assertion On dilution, the equivalent as well as molar conductivity of solution increases. Reason With dilution, the number of current carrying particles per \[c{{m}^{3}}\] increases.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 98) Assertion 2-butenal lacks enolisable H-atom, \[\alpha \] to carbonyl group, still it has sufficient acidic character. Reason The conjugate base of 2-butenal is stabilized by resonance.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 99) Assertion When NO reacts with \[\text{FeS}{{\text{O}}_{4}},\]a brown colored complex is formed. Reason In the complex, the coordination number of Fe is +6.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 100) Assertion In the Lyman series of H-spectra, the maximum wavelength of lines is 121.65 nm. Reason Wavelength is maximum if there is transition from the very next level.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 101) Who coined the term gene?
A)
Johanssen
done
clear
B)
Watson
done
clear
C)
Morgan
done
clear
D)
Williamson
done
clear
View Answer play_arrow
question_answer 102) Protein coat of a virus enclosing nucleic acid is called
A)
plasmid
done
clear
B)
capsid
done
clear
C)
vector
done
clear
D)
genome
done
clear
View Answer play_arrow
question_answer 103) Highest number of antibiotics are produced by
A)
Bacillus
done
clear
B)
Penicillium
done
clear
C)
Streptomyces
done
clear
D)
Cephalosporium
done
clear
View Answer play_arrow
question_answer 104) Which of the following is stained using carmine?
A)
Bacteria
done
clear
B)
Diatoms
done
clear
C)
Chromosomes
done
clear
D)
Viruses
done
clear
View Answer play_arrow
question_answer 105) A mature pollen grain of Pinus has
A)
2 cells
done
clear
B)
3 cells
done
clear
C)
4 cells
done
clear
D)
5 cells
done
clear
View Answer play_arrow
question_answer 106) Polyploidy can be induced by the application of
A)
auxin
done
clear
B)
kinetin
done
clear
C)
colchicine
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 107) Quantasomes are present in
A)
chloroplast
done
clear
B)
mitochondria
done
clear
C)
Golgi body
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 108) In mitochondria, enzyme cytochrome oxidase is present in
A)
outer membrane
done
clear
B)
perimitochondrial space
done
clear
C)
inner membrane
done
clear
D)
matrix
done
clear
View Answer play_arrow
question_answer 109) Which of the following bio-engineered bacteria is utilized for cleaning of marine oil slicks?
A)
Escherichia coli
done
clear
B)
Pseudomonas syringae
done
clear
C)
Pseudomonas putida
done
clear
D)
Rhizoctonia solani
done
clear
View Answer play_arrow
question_answer 110) Green potatoes are toxic due to
A)
phytoalexins
done
clear
B)
solanin
done
clear
C)
triazine
done
clear
D)
hormones
done
clear
View Answer play_arrow
question_answer 111) Cells obtained from cancerous tumours are known as
A)
hyrbridomas
done
clear
B)
myelomas
done
clear
C)
lymphocytes
done
clear
D)
monoclonal cells
done
clear
View Answer play_arrow
question_answer 112) The plant of Triticum aestivum is
A)
haploid
done
clear
B)
diploid
done
clear
C)
tetraploid
done
clear
D)
hexaploid
done
clear
View Answer play_arrow
question_answer 113) Which of the following is a total root parasite?
A)
Cuscuta
done
clear
B)
Rafflesia
done
clear
C)
Santalum
done
clear
D)
Monotrapa
done
clear
View Answer play_arrow
question_answer 114) Which of the following tissues consist of living cells?
A)
Vessels
done
clear
B)
Tracheids
done
clear
C)
Companion cell
done
clear
D)
Sclerenchyma
done
clear
View Answer play_arrow
question_answer 115) Which is a useful product of epidermal origin ?
A)
Saffron
done
clear
B)
Cotton fibres
done
clear
C)
Clove
done
clear
D)
Jute
done
clear
View Answer play_arrow
question_answer 116) Fern spores are usually
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 117) When pollen tube enters through micropyle, the process is called
A)
porogamy
done
clear
B)
chalazogamy
done
clear
C)
mesogamy
done
clear
D)
apogamy
done
clear
View Answer play_arrow
question_answer 118) Outer wall of pollen grain is made up of
A)
cellulose
done
clear
B)
sporopollenin
done
clear
C)
pectocellulose
done
clear
D)
lignin
done
clear
View Answer play_arrow
question_answer 119) Nucleotides are formed by
A)
purine, sugar and phosphate
done
clear
B)
purine, pyrimidine and phosphate
done
clear
C)
purine, pyrimidine, sugar and phosphate
done
clear
D)
pyrimidine, sugar and phosphate
done
clear
View Answer play_arrow
question_answer 120) DNA replication occurs in
A)
\[{{G}_{1}}\]-phase
done
clear
B)
S-phase
done
clear
C)
\[{{G}_{2}}\]-phase
done
clear
D)
M-phase
done
clear
View Answer play_arrow
question_answer 121) Which of the following plant cells is not surrounded by a cell wall ?
A)
Root hair cell
done
clear
B)
Stem hair cell
done
clear
C)
Gamete cell
done
clear
D)
Bacterial cell
done
clear
View Answer play_arrow
question_answer 122) Which of the following cell organelles stores hydrolytic enzymes ?
A)
Centriole
done
clear
B)
Lysosome
done
clear
C)
Chromoplast
done
clear
D)
Chloroplast
done
clear
View Answer play_arrow
question_answer 123) A monocarpic plant is one, which
A)
has only one carpel
done
clear
B)
flowers once in a life-time
done
clear
C)
produces only one seed
done
clear
D)
produces only one fruit
done
clear
View Answer play_arrow
question_answer 124) AIDS virus contains
A)
RNA with protein
done
clear
B)
DNA with protein
done
clear
C)
DNA without protein
done
clear
D)
DNA only
done
clear
View Answer play_arrow
question_answer 125) Calyptra develops from
A)
venter wall of archegonium
done
clear
B)
outgrowth of gametophyte
done
clear
C)
neck wall of archegonium
done
clear
D)
paraphysis of the archegonial branch
done
clear
View Answer play_arrow
question_answer 126) Protonema is the stage in the life-cycle of
A)
Cycas
done
clear
B)
Funaria
done
clear
C)
Selaginella
done
clear
D)
Mucor
done
clear
View Answer play_arrow
question_answer 127) A ferm differs from a moss in having
A)
swimming archegonia
done
clear
B)
swimming antherozoids
done
clear
C)
independent gametophytes
done
clear
D)
independent sporophytes
done
clear
View Answer play_arrow
question_answer 128) Female cone of Pinus is a
A)
modified needles
done
clear
B)
modified long shoot
done
clear
C)
modified dwarf shoot
done
clear
D)
modified scale
done
clear
View Answer play_arrow
question_answer 129) Development of an embryo without fertilization is called as
A)
apomixis
done
clear
B)
polyembryony
done
clear
C)
parthenocarpy
done
clear
D)
parthenogenesis
done
clear
View Answer play_arrow
question_answer 130) Which of the following floral parts forms pericarp after fertilization ?
A)
Nucellus
done
clear
B)
Outer integument
done
clear
C)
Ovary wall
done
clear
D)
Inner integument
done
clear
View Answer play_arrow
question_answer 131) Prothallus of the fern produces
A)
spores
done
clear
B)
gametes
done
clear
C)
Both (a) and (b)
done
clear
D)
cones
done
clear
View Answer play_arrow
question_answer 132) Which of the following cell organelles is associated with photorespiration?
A)
Mitochondria
done
clear
B)
Peroxisome
done
clear
C)
Chloroplast
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 133) The thickness of unit membrane is
A)
\[20\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[35\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[55\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[75\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 134) Chromosomes are arranged along the equator during
A)
prophase
done
clear
B)
metaphase
done
clear
C)
anaphase
done
clear
D)
telophase
done
clear
View Answer play_arrow
question_answer 135) Width of the DNA molecule is
A)
\[15\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[20\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[25\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[34\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 136) In gymnosperms, the ovule is naked because
A)
ovary wall is absent
done
clear
B)
integuments are absent
done
clear
C)
perianth is absent
done
clear
D)
nucellus is absent
done
clear
View Answer play_arrow
question_answer 137) In Funaria capsule, dispersal of spores takes place through
A)
peristomial teeth
done
clear
B)
annulus
done
clear
C)
calyptra
done
clear
D)
operculum
done
clear
View Answer play_arrow
question_answer 138) Crossing over occurs during
A)
leptotene
done
clear
B)
diplotene
done
clear
C)
pachytene
done
clear
D)
zygotene
done
clear
View Answer play_arrow
question_answer 139) Genes are made up of
A)
histones
done
clear
B)
hydrocarbons
done
clear
C)
polynucleotides
done
clear
D)
lipoproteins
done
clear
View Answer play_arrow
question_answer 140) The site of photosynthesis in blue-green algae is
A)
chromatophores
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
root hair
done
clear
View Answer play_arrow
question_answer 141) Viral infection is usually absent in
A)
phloem cells
done
clear
B)
xylem cells
done
clear
C)
pith cells
done
clear
D)
apical meristem
done
clear
View Answer play_arrow
question_answer 142) Clamp connections are found in
A)
Phycomycetes
done
clear
B)
Ascomycetes
done
clear
C)
Basidiomycetes
done
clear
D)
Deuteromycetes
done
clear
View Answer play_arrow
question_answer 143) Which of the following characters is related with telophase?
A)
Formation of nuclear membrane
done
clear
B)
Formation of nucleolus
done
clear
C)
Elongation of chromosome
done
clear
D)
Formation of two daughter nuclei
done
clear
View Answer play_arrow
question_answer 144) In case of incomplete dominance, what will be the phenotypic ratio of \[{{F}_{2}}\] generation?
A)
3 : 1
done
clear
B)
1 : 2 : 1
done
clear
C)
1 : 1 : 1 : 1
done
clear
D)
2 : 2
done
clear
View Answer play_arrow
question_answer 145) Which of the following does not contain DNA?
A)
Mitochondria
done
clear
B)
Chloroplast
done
clear
C)
Peroxysome
done
clear
D)
Nucleus
done
clear
View Answer play_arrow
question_answer 146) Genes exhibiting multiple effects are known as
A)
complementary genes
done
clear
B)
pleiotropic genes
done
clear
C)
cistrons
done
clear
D)
pseudogenes
done
clear
View Answer play_arrow
question_answer 147) Who coined the term cistron ?
A)
Muller
done
clear
B)
Benzer
done
clear
C)
Khorana
done
clear
D)
Sutton
done
clear
View Answer play_arrow
question_answer 148) Which of the following is responsible for the origin of lysosome ?
A)
Chloroplast
done
clear
B)
Mitochondria
done
clear
C)
Golgi body
done
clear
D)
Ribosome
done
clear
View Answer play_arrow
question_answer 149) In Selaginella, trabeculae are the modification of
A)
epidermal cells
done
clear
B)
cortical cells
done
clear
C)
endodermal cells
done
clear
D)
pericycle cells
done
clear
View Answer play_arrow
question_answer 150) Tonoplast is a membrane, which surrounds
A)
ribosome
done
clear
B)
mitochondria
done
clear
C)
vacuole
done
clear
D)
cytoplasm
done
clear
View Answer play_arrow
question_answer 151) Which shows polymorphism ?
A)
Physalia
done
clear
B)
Trypanosoma
done
clear
C)
Termite
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 152) Secondary radial symmetry is found in
A)
Cnidaria
done
clear
B)
Jelly fish
done
clear
C)
Echinodermata
done
clear
D)
Hemichordata
done
clear
View Answer play_arrow
question_answer 153) Basic unit of classification is
A)
genus
done
clear
B)
species
done
clear
C)
order
done
clear
D)
class
done
clear
View Answer play_arrow
question_answer 154) Connecting link between Annelida and Mollusca is
A)
Peripatus
done
clear
B)
Lepidosiren
done
clear
C)
Neopilina
done
clear
D)
Protopterus
done
clear
View Answer play_arrow
question_answer 155) Select incorrect pair
A)
Porifera - choanocytes
done
clear
B)
Coelenterata - nematocysts
done
clear
C)
Annelida - segmentation
done
clear
D)
Monera - eukaryote
done
clear
View Answer play_arrow
question_answer 156) Bilateral symmetry, metameric segmentation, coelom and open circulatory are the features of
A)
Annelida
done
clear
B)
Arthropoda
done
clear
C)
Mollusca
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 157) Ancestor of man who first stood erect was
A)
Australopithecus
done
clear
B)
Cro-magnon
done
clear
C)
Java-ape man
done
clear
D)
Peking man
done
clear
View Answer play_arrow
question_answer 158) Core zone, buffer zone and manipulation zone are found in
A)
national park
done
clear
B)
sanctuary
done
clear
C)
tiger reserve
done
clear
D)
biosphere reserve
done
clear
View Answer play_arrow
question_answer 159) Which insecticide is more hazardous to human health ?
A)
Rotenone
done
clear
B)
Pyrethrum
done
clear
C)
DDT
done
clear
D)
Humulin
done
clear
View Answer play_arrow
question_answer 160) Universal donor is
A)
\[O\,R{{h}^{+}}\]
done
clear
B)
\[O\,R{{h}^{-}}\]
done
clear
C)
\[AB\,R{{h}^{+}}\]
done
clear
D)
\[AB\,R{{h}^{-}}\]
done
clear
View Answer play_arrow
question_answer 161) One of these is not concerned with wild-life conservation
A)
IVF
done
clear
B)
IUCN
done
clear
C)
WWF
done
clear
D)
IBWL
done
clear
View Answer play_arrow
question_answer 162) Largest tiger population is found in
A)
Sunderban national park
done
clear
B)
Corbett national park
done
clear
C)
Ranthambhor national park
done
clear
D)
Kanha national park
done
clear
View Answer play_arrow
question_answer 163) Genetic material found in Human Immunodeficiency Virus (HIV) is
A)
double stranded RNA
done
clear
B)
single stranded RNA
done
clear
C)
double stranded DNA
done
clear
D)
single stranded DNA
done
clear
View Answer play_arrow
question_answer 164) Gigantism and acromegaly are due to
A)
hypothyroidism
done
clear
B)
hyperthyroidism
done
clear
C)
hypopituitarism
done
clear
D)
hyperpituitarism
done
clear
View Answer play_arrow
question_answer 165) If a child is of O blood group and his father is of B blood group, the genotype of father is
A)
\[{{I}^{o}}{{I}^{o}}\]
done
clear
B)
\[{{I}^{A}}\,{{I}^{B}}\]
done
clear
C)
\[{{I}^{o}}\,{{I}^{B}}\]
done
clear
D)
\[{{I}^{o}}\,{{I}^{A}}\]
done
clear
View Answer play_arrow
question_answer 166) Spermatogenesis is under the regulatory influence of
A)
ADH
done
clear
B)
FSH
done
clear
C)
LH
done
clear
D)
STH
done
clear
View Answer play_arrow
question_answer 167) Which of the following can be controlled by using biopesticides?
A)
Insects
done
clear
B)
Diseases
done
clear
C)
Weeds
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 168) Which hormone is secreted in a woman if pregnancy has occurred ?
A)
Estrogen
done
clear
B)
Progesterone
done
clear
C)
Luteinizing hormone
done
clear
D)
Chorionic gonadotropin
done
clear
View Answer play_arrow
question_answer 169) Product of biotechnology is
A)
transgenic crops (GM crops)
done
clear
B)
humulin
done
clear
C)
biofertilizer
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 170) Phase common in aerobic and anaerobic respiration is
A)
Krebs cycle
done
clear
B)
glycolysis
done
clear
C)
glycogenolysis
done
clear
D)
ETS
done
clear
View Answer play_arrow
question_answer 171) Oxyntic cells secrete
A)
\[HCl\]
done
clear
B)
trypsin
done
clear
C)
\[NaOH\]
done
clear
D)
pepsinogen
done
clear
View Answer play_arrow
question_answer 172) Menstruation is due to sudden
A)
reduction of FSH
done
clear
B)
increase of LH
done
clear
C)
reduction in estrogen and progesterone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 173) Correctly matched set of phylum, class and example is
A)
Protozoa-Mastigophora-Entamoeba
done
clear
B)
Mollusca-Bivalvia-Pinctata
done
clear
C)
Arthropoda-Diplopoda-Scolopendra
done
clear
D)
Chordata-Cyclostomata-Phrynosoma
done
clear
View Answer play_arrow
question_answer 174) Urea synthesis occurs in
A)
kidney
done
clear
B)
liver
done
clear
C)
brain
done
clear
D)
muscles
done
clear
View Answer play_arrow
question_answer 175) Which is common to kidney and skeleton in mammals ?
A)
Cortex
done
clear
B)
Medulla
done
clear
C)
Pelvis
done
clear
D)
Radius
done
clear
View Answer play_arrow
question_answer 176) Which is regarded as urinary bladder of embryo ?
A)
Amnion
done
clear
B)
Allantois
done
clear
C)
Chorion
done
clear
D)
Yolk sac
done
clear
View Answer play_arrow
question_answer 177) Deficiency of vitamin B, 2 causes
A)
cheilosis
done
clear
B)
thalassemia
done
clear
C)
beri-beri
done
clear
D)
pernicious anaemia
done
clear
View Answer play_arrow
question_answer 178) Blood is a kind of
A)
areolar tissue
done
clear
B)
connective tissue
done
clear
C)
fluid connective tissue
done
clear
D)
reticular connective tissue
done
clear
View Answer play_arrow
question_answer 179) Which of these is used to control human population ?
A)
Estrogen + progesterone
done
clear
B)
IUCD and MTP
done
clear
C)
Tubectomy and vasectomy
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 180) Addiction to alcohol causes
A)
cirrhosis
done
clear
B)
epilepsy
done
clear
C)
neurosis
done
clear
D)
psychosis
done
clear
View Answer play_arrow
question_answer 181) The most primitive vertebrates are
A)
Ostracoderms
done
clear
B)
Cephalochordates
done
clear
C)
placoderms
done
clear
D)
Cyclostomes
done
clear
View Answer play_arrow
question_answer 182) Change in the number of body parts is called
A)
continuous variation
done
clear
B)
discontinuous variation
done
clear
C)
meristic variation
done
clear
D)
substantive variation
done
clear
View Answer play_arrow
question_answer 183) Which has an additional X chromosome ?
A)
Turners syndrome
done
clear
B)
Klinefelters syndrome
done
clear
C)
Super female
done
clear
D)
Downs syndrome
done
clear
View Answer play_arrow
question_answer 184) Origin of life occurred in
A)
Precambrian
done
clear
B)
Coenozoic
done
clear
C)
Palaeozoic
done
clear
D)
Mesozoic
done
clear
View Answer play_arrow
question_answer 185) Branch of zoology dealing with the study of fishes is called
A)
Arthrology
done
clear
B)
Ichthyology
done
clear
C)
Saurology
done
clear
D)
Herpetology
done
clear
View Answer play_arrow
question_answer 186) Theory of continuity of germplasm was propounded by
A)
Mendel
done
clear
B)
Lamarck
done
clear
C)
Weismann
done
clear
D)
Haeckel
done
clear
View Answer play_arrow
question_answer 187) Who discovered oocysts in the stomach of female Anopheles ?
A)
Ronald Ross
done
clear
B)
Charles Lavern
done
clear
C)
Golgi
done
clear
D)
Lamble
done
clear
View Answer play_arrow
question_answer 188) Epimysium, perimysium and endomysium are found in
A)
nerve
done
clear
B)
blood vessel
done
clear
C)
striated muscle
done
clear
D)
uterus
done
clear
View Answer play_arrow
question_answer 189) Endothelium is made up of
A)
squamous cells
done
clear
B)
cuboidal cells
done
clear
C)
columnar cells
done
clear
D)
stratified epithelium
done
clear
View Answer play_arrow
question_answer 190) Bipolar neurons occur in
A)
vertebrate embryos
done
clear
B)
retina of eye
done
clear
C)
brain and spinal cord
done
clear
D)
skeletal muscles
done
clear
View Answer play_arrow
question_answer 191) Glissons capsules are found, in which organ of mammals ?
A)
Stomach
done
clear
B)
Kidney
done
clear
C)
Testis
done
clear
D)
Liver
done
clear
View Answer play_arrow
question_answer 192) Production of glucose from amino acids, fatty acids and glycerol is called
A)
glycogenesis
done
clear
B)
gluconeogenesis
done
clear
C)
glycogenolysis
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 193) Which is called Hamburger shift?
A)
Hydrogen shift
done
clear
B)
Bicarbonate shift
done
clear
C)
Chloride shift
done
clear
D)
Sodium shift
done
clear
View Answer play_arrow
question_answer 194) Papillary muscles are found in mammalian
A)
auricles
done
clear
B)
ventricles
done
clear
C)
pinna
done
clear
D)
eyes
done
clear
View Answer play_arrow
question_answer 195) Which foramen is paired in mammalian brain?
A)
Foramen of Luschka
done
clear
B)
Foramen of Magendie
done
clear
C)
Foramen of Monro
done
clear
D)
Inter-ventricular foramen
done
clear
View Answer play_arrow
question_answer 196) Assertion With few exceptions tropics harbour more species than temperate or polar areas. Reason Species diversity decreases as we move away from the equator towards the poles.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 197) Assertion Reduction division occurs in anaphase-I. So there is no need of meiosis. Reason Meiosis-II occurs to separate homologous chromosomes.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 198) Assertion Allelopathy is a form of ammensalism that occurs in plants. Reason Association of rooting plants with fungal hyphae, is an important example of ammensalism.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 199) Assertion Generally, a woman do not conceive during lactation period. Reason The hormone prolactin initates and maintain lactation in a woman.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 200) Assertion The imbalance in concentration of sodium and potassium ions and proteins generates the resting potential. Reason To maintain unequal distribution of sodium and potassium, neurons use electrical energy.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
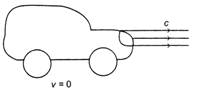
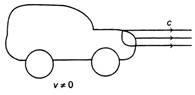 In figure, one car is at rest and velocity of light from head light is c, then velocity of light from head light for the moving car at velocity v, would be
In figure, one car is at rest and velocity of light from head light is c, then velocity of light from head light for the moving car at velocity v, would be