question_answer 1) How many seconds are there in a light fermi?
A)
\[{{10}^{-15}}g\]
done
clear
B)
\[3.0\,\times {{10}^{8}}s\]
done
clear
C)
\[3.33\,\times {{10}^{-24}}s\]
done
clear
D)
\[3.3\,\times {{10}^{-7}}s\]
done
clear
View Answer play_arrow
question_answer 2) A machine is delivering constant power to drive a body along a straight line. What is the relation between the distance travelled by the body against time?
A)
\[{{s}^{2}}\propto {{t}^{3}}\]
done
clear
B)
\[{{s}^{2}}\propto {{t}^{-3}}\]
done
clear
C)
\[{{s}^{3}}\propto {{t}^{2}}\]
done
clear
D)
\[s\propto {{t}^{3}}\]
done
clear
View Answer play_arrow
question_answer 3) The square of resultant of two equal forces is three times their product. Angle between the forces is
A)
\[\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{4}\]
done
clear
D)
\[\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 4) An object placed on a ground is in stable equilibrium. If the object is given a slight push then initially the position of centre of gravity
A)
moves nearer to ground
done
clear
B)
rises higher above the ground
done
clear
C)
remains as such
done
clear
D)
may remain at same level
done
clear
View Answer play_arrow
question_answer 5) How much work must be done by a force on 50 kg body in order to accelerate it from rest to 20 m/s in 10 s ?
A)
\[{{10}^{3}}J\]
done
clear
B)
\[{{10}^{4}}J\]
done
clear
C)
\[2\times {{10}^{3}}J\]
done
clear
D)
\[4\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 6) Moment of inertia of circular loop of radius R about the axis of rotation parallel to horizontal diameter at a distance R/2 from it is
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{1}{2}M{{R}^{2}}\]
done
clear
C)
\[2M{{R}^{2}}\]
done
clear
D)
\[\frac{3}{4}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 7) What will happen to the weight of the body at the South Pole, if the earth stops rotating about its polar axis?
A)
No change
done
clear
B)
Increases
done
clear
C)
Decreases but does not become zero
done
clear
D)
Reduces to zero
done
clear
View Answer play_arrow
question_answer 8) A beam of metal supported at the two ends is loaded at the centre. The depression at the centre is proportional to
A)
\[{{Y}^{2}}\]
done
clear
B)
\[Y\]
done
clear
C)
\[\frac{1}{Y}\]
done
clear
D)
\[\frac{1}{{{Y}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 9) A common hydrometer reads specific gravity of liquids. Compared to the 1.6 mark of the stem the mark 1.5 will be
A)
upwards
done
clear
B)
downwards
done
clear
C)
in the same place
done
clear
D)
may be upward or downward depending upon the hydrometer
done
clear
View Answer play_arrow
question_answer 10) A balloon contains \[500\,\,{{m}^{3}}\] of Heat \[27{}^\circ C\] and 1 atmospheric pressure. The volume of Heat \[-3{}^\circ C\] and 0.5 atmospheric pressure will be
A)
\[700\,{{m}^{3}}\]
done
clear
B)
\[900\,{{m}^{3}}\]
done
clear
C)
\[1000\,{{m}^{3}}\]
done
clear
D)
\[500\,{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 11) Which of the following is different from others?
A)
Wavelength
done
clear
B)
Velocity
done
clear
C)
Frequency
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 12) Two pendulums have time periods T and 5T/4. They starts SHM at the same time from the mean position. What will be the phase difference between them after the bigger pendulum completed one oscillation?
A)
\[45{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 13) A balloon is filled with hydrogen. For sound waves, this balloon behaves like
A)
a converging lens
done
clear
B)
a diverging lens
done
clear
C)
a concave mirror
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 14) Each of the two point charges are doubled and their distance is halved. Force of interaction becomes n times, where n is
A)
4
done
clear
B)
1
done
clear
C)
1/16
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 15) Two soap bubbles have radii in the ratio of 2:1. What is the ratio of excess pressures inside them?
A)
1 : 2
done
clear
B)
1 : 4
done
clear
C)
2 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 16) The phenomenon of Brownian movement may be taken as evidence of
A)
kinetic theory of matter
done
clear
B)
EMT of radiation
done
clear
C)
corpuscular theory of light
done
clear
D)
photoelectric phenomenon
done
clear
View Answer play_arrow
question_answer 17) Two sound waves of slightly different frequencies propagating in the same direction produce beats due to
A)
interference
done
clear
B)
diffraction
done
clear
C)
reflection
done
clear
D)
refraction
done
clear
View Answer play_arrow
question_answer 18) An ice block floats in a liquid whose density is less than water. A part of block is outside the liquid. When whole of ice has melted, the liquid level will
A)
rise
done
clear
B)
go down
done
clear
C)
remain same
done
clear
D)
first rise then go down
done
clear
View Answer play_arrow
question_answer 19) Two bodies of different masses of 2 kg and 4 kg moving with velocities 2 m/s and 10 m/s towards each other due to mutual gravitational attraction. What is the velocity of their centre of mass?
A)
5 m/s
done
clear
B)
6 m/s
done
clear
C)
8 m/s
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 20) Given that the displacement of an oscillating particle is given by y = A sin (Bx + Ct + D). The dimensional formula for (ABCD) is
A)
\[\left[ {{M}^{0}}{{L}^{-1}}{{T}^{0}} \right]\]
done
clear
B)
\[\left[ {{M}^{0}}{{L}^{0}}{{T}^{-1}} \right]\]
done
clear
C)
\[\left[ {{M}^{0}}{{L}^{-1}}{{T}^{-1}} \right]\]
done
clear
D)
\[\left[ {{M}^{0}}{{L}^{0}}{{T}^{0}} \right]\]
done
clear
View Answer play_arrow
question_answer 21) Two waves having intensities in the ratio of 9:1 produce interference. The ratio of maximum to minimum intensity is equal to
A)
10: 8
done
clear
B)
9 : 1
done
clear
C)
4 : 1
done
clear
D)
2:1
done
clear
View Answer play_arrow
question_answer 22) Four wires each of same length, diameter and material are connected to each other to form a square. If the resistance of each wire is R, then equivalent resistance across the opposite comers is
A)
R
done
clear
B)
R/2
done
clear
C)
R/4
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 23) An electric motor runs on DC source of emf 200 V and draws a current of 10 A. If the efficiency be 40% then the resistance of armature is
A)
\[2\Omega \]
done
clear
B)
\[8\Omega \]
done
clear
C)
\[12\Omega \]
done
clear
D)
\[16\Omega \]
done
clear
View Answer play_arrow
question_answer 24) A capacitor having capacity of 2.0 (\[\mu \]F is charged to 200 V and then the plates of the capacitor are connected to a resistance wire. The heat produced in joule will be
A)
\[2\times {{10}^{-2}}\]
done
clear
B)
\[4\times {{10}^{-2}}\]
done
clear
C)
\[4\times {{10}^{4}}\]
done
clear
D)
\[4\times {{10}^{10}}\]
done
clear
View Answer play_arrow
question_answer 25) A voltmeter of range 2V and resistance 300 Q cannot be converted into ammeter of range
A)
1 A
done
clear
B)
1 mA
done
clear
C)
100 mA
done
clear
D)
10 mA
done
clear
View Answer play_arrow
question_answer 26) If a magnet is suspended at angle \[30{}^\circ \] to the magnetic meridian, the dip needle makes angle of \[45{}^\circ \] with the horizontal. The real dip is
A)
\[{{\tan }^{-1}}\,\left( \sqrt{3}\,/2 \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \sqrt{3} \right)\]
done
clear
C)
\[{{\tan }^{-1}}\,\left( \sqrt{3}\,/2 \right)\]
done
clear
D)
\[{{\tan }^{-1}}\left( 2/\sqrt{3} \right)\]
done
clear
View Answer play_arrow
question_answer 27) Which quantity is increased in step-down transformer?
A)
Current
done
clear
B)
Voltage
done
clear
C)
Power
done
clear
D)
Frequency
done
clear
View Answer play_arrow
question_answer 28) The ratio of intensity at the centre of a bright fringe to the intensity at a point distant one fourth of the distance between two successive bright fringes will be
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 29) Which has more luminous efficiency?
A)
A 40 W bulb
done
clear
B)
A 40 W fluorescent tube
done
clear
C)
Both have same
done
clear
D)
Cannot say
done
clear
View Answer play_arrow
question_answer 30) When a ray of light enters from one medium to another, then its velocity in second medium becomes double. The maximum value of angle of incidence, so that total internal reflection may not take place will be
A)
\[60{}^\circ \]
done
clear
B)
\[180{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 31) What should be the velocity of an electron so that its momentum becomes equal to that of a photon of wavelength \[5200\overset{\text{o}}{\mathop{\text{A}}}\,\]?
A)
700 m/s
done
clear
B)
1000 m/s
done
clear
C)
1400m/s
done
clear
D)
2800 m/s
done
clear
View Answer play_arrow
question_answer 32) A radioactive element has half-life period of 600 years. After 3000 years, what amount will remain?
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{16}\]
done
clear
C)
\[\frac{1}{8}\]
done
clear
D)
\[\frac{1}{32}\]
done
clear
View Answer play_arrow
question_answer 33) Beyond which frequency, the ionosphere bends any incident electromagnetic radiation but do not reflect it back towards the earth?
A)
50 MHz
done
clear
B)
40 MHz
done
clear
C)
30 MHz
done
clear
D)
20 MHz
done
clear
View Answer play_arrow
question_answer 34) In intrinsic semiconductor at room temperature number of electrons and holesare
A)
equal
done
clear
B)
zero
done
clear
C)
unequal
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 35) The unit of thermal conductance is
A)
\[W{{K}^{-1}}\]
done
clear
B)
\[J{{K}^{-1}}\]
done
clear
C)
WK
done
clear
D)
JK
done
clear
View Answer play_arrow
question_answer 36) The value of P so that the vectors\[2\mathbf{\hat{i}}-\mathbf{\hat{j}}+\mathbf{\hat{k}}\],\[\mathbf{\hat{i}}-2\mathbf{\hat{j}}+3\mathbf{\hat{k}}\]and \[3\mathbf{\hat{i}}-P\mathbf{\hat{j}}+5\mathbf{\hat{k}}\] are coplanar should be
A)
16
done
clear
B)
-4
done
clear
C)
4
done
clear
D)
-8
done
clear
View Answer play_arrow
question_answer 37) A capacitor of capacitance C has charge Q and stored energy is W. If the charge is increased to 2Q the stored energy will be
A)
\[\frac{W}{4}\]
done
clear
B)
\[\frac{W}{2}\]
done
clear
C)
2 W
done
clear
D)
4 W
done
clear
View Answer play_arrow
question_answer 38) Pure silicon at 300 K has equal electron \[{{n}_{e}}\] and hole (\[{{n}_{h}}\]) concentration of \[1.5\times {{10}^{16}}{{m}^{-3}}\]. Doping by indium increases \[{{n}_{h}}\] to \[4.5\times {{10}^{22}}{{m}^{-3}}\]. The \[{{n}_{e}}\] in the doped silicon is
A)
\[9\times {{10}^{5}}\]
done
clear
B)
\[5\times {{10}^{9}}\]
done
clear
C)
\[2.25\times {{10}^{11}}\]
done
clear
D)
\[3\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 39) A cylindrical conductor is placed near another positively charged conductor. The net charge acquired by the cylindrical conductor will be
A)
positive only
done
clear
B)
negative only
done
clear
C)
zero
done
clear
D)
either positive or negative
done
clear
View Answer play_arrow
question_answer 40) If the unit of force is 1 kilo newton, the length is 1 km and time 100 s, what will be the unit of mass?
A)
1, 000 kg
done
clear
B)
1 kg
done
clear
C)
10, 000 kg
done
clear
D)
100 kg
done
clear
View Answer play_arrow
question_answer 41) The maximum tension which an inextensible ring of mass 0.1 kg/m can bear is 10 N. The maximum velocity in m/s with which it can be rotated is
A)
10
done
clear
B)
\[\sqrt{10}\]
done
clear
C)
20
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 42) If there were a reduction in gravitational effect, which of the following forces do you think would change in some respect?
A)
Magnetic force
done
clear
B)
Electrostatic force
done
clear
C)
Viscous force
done
clear
D)
Archimedes uplift
done
clear
View Answer play_arrow
question_answer 43) The breaking force for a wire of diameter D of a material is F. The breaking force for a wire of the same material of radius D is
A)
F
done
clear
B)
2 F
done
clear
C)
\[\frac{F}{4}\]
done
clear
D)
4F
done
clear
View Answer play_arrow
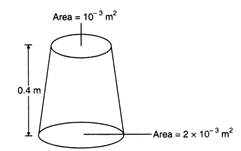
question_answer 44)
A uniformly tapering vessel is filled with a liquid of density \[900\,kg/{{m}^{3}}\]. The force that acts on the base of the vessel due to the liquid is \[(g=10\,m/{{s}^{2}})\]
A)
3.6N
done
clear
B)
7.2N
done
clear
C)
9.0N
done
clear
D)
14.4N
done
clear
View Answer play_arrow
question_answer 45) If pressure of a gas contained in a closed vessel is increased by 0.4% when heated by \[1{}^\circ C\], its initial temperature must be
A)
250 K
done
clear
B)
\[250{}^\circ C\]
done
clear
C)
2500 K
done
clear
D)
\[25{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 46) Assertion: A body is projected vertically upwards with velocity 10 ms-1. It reaches the maximum vertical height h in time t. In the height covered is\[\frac{3h}{4}\] Reason :\[t=\sqrt{\frac{2h}{g}}\,and\,\,{{v}^{2}}={{u}^{2}}+2gh\]
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 47) Assertion: A person in the lift experiences weightless, when the lift starts going down with some acceleration. Reason: The gravitational acceleration gets reduced.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 48) Assertion: A rain drop after falling through some height attains a constant velocity. Reason: At constant velocity the viscous drag is just equal to its weight.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 49) Assertion: The torque on the coil is maximum, when coil is suspended in a radial magnetic field. Reason: The torque tends to rotate the coil about its own axis.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 50)
Assertion: It one gram of ice is mixed with 1 g of water at
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 51) Ethyl acetate is obtained when methyl magnesium bromide reacts with
A)
ethyl formate
done
clear
B)
ethyl chloroformate
done
clear
C)
acetyl chloride
done
clear
D)
carbon dioxide
done
clear
View Answer play_arrow
question_answer 52) The most stable hydride is
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[As{{H}_{3}}\]
done
clear
D)
\[Sb{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 53) The ratio of amounts of \[{{\text{H}}_{\text{2}}}\text{S}\]needed to precipitate all the metal ions from 100 mL of \[\text{1}\,\text{M}\,\text{AgN}{{\text{O}}_{3}}\]and 100 mL of \[\text{1}\,\text{M}\,\text{CuS}{{\text{O}}_{4}}\] will be
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
2 : 1
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 54) If the electronegativity difference between two atoms A and B is 2.0, then the percentage of covalent character in the molecule is
A)
54%
done
clear
B)
46%
done
clear
C)
23%
done
clear
D)
72%
done
clear
View Answer play_arrow
question_answer 55) Which of the following reaction defines \[\Delta H_{f}^{o}?\]
A)
\[{{C}_{(diamond)}}+{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\]
done
clear
B)
\[\frac{1}{2}{{H}_{2}}(g)+\frac{1}{2}{{F}_{2}}(g)\xrightarrow{{}}HF(g)\]
done
clear
C)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\xrightarrow{{}}2N{{H}_{3}}(g)\]
done
clear
D)
\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 56) Formaldehyde polymerizes to form glucose according to the reaction \[6HCHO{{C}_{6}}{{H}_{12}}{{O}_{6}}\] The theoretically computed equilibrium constant for this reaction is found to be \[6\times {{10}^{22}}.\] If 1 M solution of glucose dissociates according to the above equilibrium, the concentration of formaldehyde in the solution will be
A)
\[1.6\times {{10}^{-2}}M\]
done
clear
B)
\[1.6\times {{10}^{-4}}M\]
done
clear
C)
\[1.6\,\times {{10}^{-6}}M\]
done
clear
D)
\[1.6\,\times {{10}^{-8}}\,M\]
done
clear
View Answer play_arrow
question_answer 57) The electronic configuration of a dipositive ion \[{{\text{M}}^{\text{2+}}}\]is 2, 8, 14 and its mass number is 56. The number of neutrons present is
A)
32
done
clear
B)
42
done
clear
C)
30
done
clear
D)
34
done
clear
View Answer play_arrow
question_answer 58) If X is the total number of collisions which a gas molecule register with others per unit time under particular conditions, then the collision frequency of the gas containing N molecules per unit volume is
A)
X/N
done
clear
B)
NX
done
clear
C)
2 NX
done
clear
D)
NX/2
done
clear
View Answer play_arrow
question_answer 59) A hypothetical reaction \[{{A}_{2}}+{{B}_{2}}\to 2AB\]follows the mechanism as given below, \[{{A}_{2}}A+A(fast)\] \[A+{{B}_{2}}\xrightarrow{{}}AB+B(slow)\] \[A+B\xrightarrow{{}}AB(fast)\] The order of the overall reaction is
A)
2
done
clear
B)
1
done
clear
C)
\[1\frac{1}{2}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 60) The mass of helium atom of mass number 4 is 4.0026 amu, while that of the neutron and proton are 1.0087 and 1.0078 respectively on the same scale. Hence, the nuclear binding energy per nucleon in the helium atom is nearly
A)
\[5MeV\]
done
clear
B)
\[7MeV\]
done
clear
C)
\[10\,MeV\]
done
clear
D)
\[14\,MeV\]
done
clear
View Answer play_arrow
question_answer 61) Which of the following statements is correct? Dielectric constant of \[{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\]
A)
increases with dilution
done
clear
B)
decreases with dilution
done
clear
C)
is unaffected on dilution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 62) For the square planar complex \[[M(a)(b)(c)(d)]\] (where, M = central metal and , Jb, c and d are monodentate ligands), the number of possible geometrical isomers are
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 63) Potash alum dissolves in water to give a/an
A)
acidic solution of \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
alkaline solution
done
clear
C)
acidic solution of \[HCl\]
done
clear
D)
neutral solution
done
clear
View Answer play_arrow
question_answer 64) The discovery of which of the following group of elements gave death blow to the Newlands law of octaves?
A)
Inert gases
done
clear
B)
Alkaline earths
done
clear
C)
Rare earths
done
clear
D)
Actinides
done
clear
View Answer play_arrow
question_answer 65) Vant Hoff factor more than unity indicates that the solute in solution has
A)
dissociated
done
clear
B)
associated
done
clear
C)
Both (a) and (b)
done
clear
D)
cannot say anything
done
clear
View Answer play_arrow
question_answer 66) How many number of atoms are there in a cube based unit cell having one atom on each comer and two atoms on each body diagonal of cube?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 67) Bleeding due to a cut can be stopped by applying ferric chloride solution in the laboratory. This is due to
A)
co-agulation of negatively charged blood particles by \[\text{F}{{\text{e}}^{\text{3+}}}\] ions
done
clear
B)
co-agulation of positively charged blood particles by\[\text{C}{{\text{l}}^{-}}\] ions
done
clear
C)
reaction taking place between ferric ions and the haemoglobin forming a complex
done
clear
D)
common element, iron, in both \[\text{FeC}{{\text{l}}_{\text{3}}}\] and haemoglobin
done
clear
View Answer play_arrow
question_answer 68) Which one of the following solutions will have highest conductivity?
A)
\[0.1\,M\,C{{H}_{3}}COOH\]
done
clear
B)
\[0.1\,M\,NaCl\]
done
clear
C)
\[0.1\,M\,KN{{O}_{3}}\]
done
clear
D)
\[0.1\,M\,HCl\]
done
clear
View Answer play_arrow
question_answer 69) One of the following metals forms a volatile compound and this property is taken advantage for its extraction. This metal is
A)
iron
done
clear
B)
nickel
done
clear
C)
cobalt
done
clear
D)
tungsten
done
clear
View Answer play_arrow
question_answer 70) If \[\text{N}{{\text{a}}^{\text{+}}}\] ion is larger than \[\text{M}{{\text{g}}^{\text{2+}}}\] ion and \[{{\text{S}}^{2-}}\] ion is larger than \[\text{C}{{\text{l}}^{-}}\] ion, which of the following will be stable soluble in water?
A)
Sodium chloride
done
clear
B)
Sodium sulphide
done
clear
C)
Magnesium chloride
done
clear
D)
Magnesium sulphide
done
clear
View Answer play_arrow
question_answer 71) Impurities of Cu and Ag from gold are removed by
A)
boiling impure gold with dil. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
B)
boiling impure gold with conc. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
electrolytically
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 72) Which of the following salt would give \[\text{S}{{\text{O}}_{\text{2}}}\] with hot and dil. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\] also decolourises \[\text{B}{{\text{r}}_{2}}\] water?
A)
\[N{{a}_{2}}S{{O}_{3}}\]
done
clear
B)
\[NaHS{{O}_{4}}\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
D)
\[N{{a}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 73) If two compounds have the same empirical formula but different molecular formulae, they must have
A)
different percentage composition
done
clear
B)
different molecular weights
done
clear
C)
same viscocity
done
clear
D)
same vapour density
done
clear
View Answer play_arrow
question_answer 74) Among the following which one has weakest carbon-halogen bond?
A)
Benzyl bromide
done
clear
B)
Bromobenzene
done
clear
C)
Vinyl bromide
done
clear
D)
Benzyl chloride
done
clear
View Answer play_arrow
question_answer 75) Petrochemicals can be used to prepare
A)
synthetic fibres
done
clear
B)
pesticides
done
clear
C)
plastics
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 76) tert-butyl methyl ether on heating with anhydrous HI in ether gives
A)
\[C{{H}_{3}}OH+{{(C{{H}_{3}})}_{3}}Cl\]
done
clear
B)
\[C{{H}_{3}}I+{{(C{{H}_{3}})}_{3}}COH\]
done
clear
C)
\[C{{H}_{3}}I+{{(C{{H}_{3}})}_{3}}Cl\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 77) The correctly reported answer of the addition of 4.523, 2.3 and 6.24 will have significant figures
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
five
done
clear
View Answer play_arrow
question_answer 78) What happens if \[\text{CC}{{\text{l}}_{\text{4}}}\] is treated with \[\text{AgN}{{\text{O}}_{\text{3}}}?\]
A)
A white ppt. of \[\text{AgCl}\] will form
done
clear
B)
\[\text{N}{{\text{O}}_{2}}\] will be evolved
done
clear
C)
\[\text{CC}{{\text{l}}_{\text{4}}}\] will dissolve in \[\text{AgN}{{\text{O}}_{\text{3}}}\]
done
clear
D)
Nothing will happen
done
clear
View Answer play_arrow
question_answer 79) \[{{\,}^{\text{23}}}\text{Na}\] is more stable isotope of Na. Find out the process by which \[_{\text{11}}^{\text{24}}\text{Na}\] can undergo radioactive decay
A)
\[\beta -\] emission
done
clear
B)
\[\alpha -\] emission
done
clear
C)
\[{{\beta }^{+}}\] emission
done
clear
D)
K electron capture
done
clear
View Answer play_arrow
question_answer 80) The heat of combustion of solid benzoic acid at constant volume is -321.30 kJ at \[\text{27}{{\,}^{\text{o}}}\text{C}\] The heat of combustion at constant pressure is
A)
\[-321.30-300\text{ }R\]
done
clear
B)
\[-321.30+300R\]
done
clear
C)
\[-321.30-150\text{ }R\]
done
clear
D)
\[-\text{ }321.30\text{ }+\text{ }900\text{ }R\]
done
clear
View Answer play_arrow
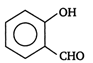
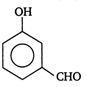
question_answer 81) In which of the following compounds ?OH group is least reactive?
A)
done
clear
B)
done
clear
C)
done
clear
D)
All are equally reactive.
done
clear
View Answer play_arrow
question_answer 82) lodoform is obtained when ethanol is heated with
A)
\[\text{KI}\] and \[aq\,KOH\]
done
clear
B)
\[{{I}_{2}}\] and \[aq\,KOH\]
done
clear
C)
\[{{I}_{2}}/aq\,KI\]
done
clear
D)
\[HI\] and \[HI{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 83) The total number of acylic isomers including the stereoisomers (geometrical and optical), with the molecular formula \[{{C}_{4}}{{H}_{7}}Cl\] is
A)
12
done
clear
B)
11
done
clear
C)
10
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 84) The alkyl halides that can be made by free radical halogenation of alkanes are
A)
\[RCl,\] and \[RBr\] but not RF or \[RI\]
done
clear
B)
\[RF,RCl\] and \[R\,Br\] but not \[RI\]
done
clear
C)
\[RF,\text{ }RCl,\text{ }RBr,\text{ }RI\]
done
clear
D)
\[RF,RCl\]and \[RI\]but \[RBr\]
done
clear
View Answer play_arrow
question_answer 85) Silica is a/an
A)
acidic flux only
done
clear
B)
gangue only
done
clear
C)
basic flux only
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 86) The nodes present in 3p-orbitals are
A)
one spherical, one planar
done
clear
B)
two spherical
done
clear
C)
two planar
done
clear
D)
one planar
done
clear
View Answer play_arrow
question_answer 87) The number of \[\alpha -\] and \[\beta -\] particles emitted in nuclear reaction \[_{90}T{{h}^{228}}\xrightarrow{{}}{{\,}_{83}}B{{i}^{212}}\] are respectively
A)
4, 1
done
clear
B)
3, 7
done
clear
C)
8, 1
done
clear
D)
4, 7
done
clear
View Answer play_arrow
question_answer 88) Two bottles A and B contains 1 M and 1 m aqueous solution of sulphuric acid respectively
A)
A is more concentrated than B
done
clear
B)
B is more concentrated than A
done
clear
C)
concentration of A is equal to concentration of B
done
clear
D)
it is not possible to compare the concentrations
done
clear
View Answer play_arrow
question_answer 89) A salt on treatment with dil. \[\text{HCl}\] gives a pungent smelling gas and a yellow precipitate. The salt gives green flame test and a yellow precipitate with potassium chromate the salt is
A)
\[NiS{{O}_{4}}\text{ }\]
done
clear
B)
\[Ba{{S}_{2}}{{O}_{3}}\]
done
clear
C)
\[Pb{{S}_{2}}{{O}_{3}}\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 90) Which of the oxide of manganese is amphoteric?
A)
\[Mn{{O}_{2}}\]
done
clear
B)
\[M{{n}_{2}}{{O}_{3}}\]
done
clear
C)
\[M{{n}_{2}}{{O}_{7}}\]
done
clear
D)
\[MnO\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following alkenes is most reactive towards cationic polymerization?
A)
\[C{{H}_{2}}=CHC{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}C=CHCl\]
done
clear
C)
\[{{H}_{2}}C=CH{{C}_{6}}{{H}_{5}}\]
done
clear
D)
\[{{H}_{2}}C=CHC{{O}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) An organic compound, \[{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{O}\]does not give a precipitate with 2, 4-dinitrophenyl hydrazine reagent and does not react with metallic sodium. It could be
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[~C{{H}_{2}}=CHC{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{2}}=CH-O-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 93) Oxidation of 1-butene with hot \[\text{KMn}{{\text{O}}_{\text{4}}}\]solution produces
A)
\[C{{H}_{3}}C{{H}_{2}}COOH\text{ }+\text{ }HCOOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH\text{ }+\text{ }C{{O}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{O}_{2}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}\text{ }C=O\text{ }+\text{ }C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 94) A mixture of 1-chlorobutane and 2-chloro- butane when treated with alcoholic KOH gives
A)
1-butene
done
clear
B)
2-butene
done
clear
C)
isobutylene
done
clear
D)
mixture of 1-butene +2-butene
done
clear
View Answer play_arrow
question_answer 95)
Out of the two compounds shown below, the vapour pressure of B at a particular temperature is expected to be
A)
higher than that of A
done
clear
B)
lower than that of B
done
clear
C)
same as that of A
done
clear
D)
can be higher lower depending upon the size of the vessel
done
clear
View Answer play_arrow
question_answer 96) Assertion : Bond energy has order like: \[C-C<C=C<C\equiv C\] Reason: Bond energy increases with increase in bond order.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 97) Assertion: \[\text{F}{{\text{e}}^{\text{3+}}}\] is more stable than \[\text{F}{{\text{e}}^{\text{2+}}}.\] Reason: \[\text{F}{{\text{e}}^{\text{2+}}}\]ions are easily oxidized to \[\text{F}{{\text{e}}^{3+}}\] ions.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 98) Assertion: Benzene reacts with \[\text{C}{{\text{H}}_{\text{3}}}\text{Cl}\] using \[\text{AlC}{{\text{l}}_{\text{3}}}\]as catalyst to form methyl benzene. This reaction is called Perkin reactions. Reason: \[\text{AlC}{{\text{l}}_{\text{3}}}\]acts as an electrophile in the reaction.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 99) Assertion: The order of reaction may be negative. Reason: In some cases, the rate of reaction decreases as the concentration of the reaction.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 100) Assertion: Fructose reduces Fehlings solution and Tollens reagent. Reaction: Fructose does not contain any aldehydic group.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 101) Correct sequence of stages in evolution of modem man/Homo sapiens is
A)
Australopithecus, Neanderthal man, Cro-magnon man, Homo erectus, modem man
done
clear
B)
Australopithecus, Homo erectus, Neanderthal man, Cro-magnon man, Modem man
done
clear
C)
Neanderthal man, Australopithecus Cro-magnon man, Homo erectus, modem man
done
clear
D)
Homo erectus, Australopithecus, Neanderthal man, Cro-magnon man, modem man
done
clear
View Answer play_arrow
question_answer 102) Allopatric speciation occurs when
A)
genetically related population inhabit widely separated geographical area
done
clear
B)
genetically unrelated population inhabit widely separated geographical area
done
clear
C)
genetically related population inhabit the same geographical area
done
clear
D)
genetically mixed population inhabit the geographical area
done
clear
View Answer play_arrow
question_answer 103) Symplast
A)
is living component of cell
done
clear
B)
consist of cytoplasm and cell membrane
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 104) Astral rays are formed of
A)
micro-filaments
done
clear
B)
Microtubules
done
clear
C)
intermediate filaments
done
clear
D)
mircrovilli
done
clear
View Answer play_arrow
question_answer 105) Which of the following cannot be isolated from plants ?
A)
Vitamin-\[{{B}_{12}}\]
done
clear
B)
Niacin
done
clear
C)
Vitamin-C
done
clear
D)
Riboflavin
done
clear
View Answer play_arrow
question_answer 106) Progesterone level falls during
A)
gestation
done
clear
B)
menopause
done
clear
C)
lactation
done
clear
D)
menstruation
done
clear
View Answer play_arrow
question_answer 107) Endangered plants species are conserved through
A)
herbarium
done
clear
B)
gene library
done
clear
C)
gene bank
done
clear
D)
reducing pollution
done
clear
View Answer play_arrow
question_answer 108) The intensity of noise pollution is measured in
A)
mach
done
clear
B)
hertz
done
clear
C)
decibels
done
clear
D)
noy
done
clear
View Answer play_arrow
question_answer 109) Which one depicts nitrogen fixation ?
A)
\[{{N}_{2}}\to N{{H}_{3}}\]
done
clear
B)
\[{{N}_{2}}\to N{{O}_{3}}\]
done
clear
C)
\[{{N}_{2}}\to A\min oacids\]
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 110) Glycolysis is
A)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}+6{{O}_{2}}\to 6C{{O}_{2}}+6{{H}_{2}}O\]
done
clear
B)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\to 2{{C}_{2}}{{H}_{5}}OH+2C{{O}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\to 2{{C}_{3}}{{H}_{4}}{{O}_{3}}+4H\]
done
clear
D)
\[{{C}_{3}}{{H}_{4}}{{O}_{3}}+NADH\to {{C}_{2}}{{H}_{5}}OH+C{{O}_{2}}+NA{{D}^{+}}\]
done
clear
View Answer play_arrow
question_answer 111) Most of the biological energy is supplied by mitochondria through
A)
breaking of proteins
done
clear
B)
reduction of NADP
done
clear
C)
breaking of sugar
done
clear
D)
oxidizing TCA substrate
done
clear
View Answer play_arrow
question_answer 112) Photolysis of water by isolated chloroplasts was demonstrated by
A)
Hill
done
clear
B)
Liebig
done
clear
C)
Von Niel
done
clear
D)
Calvin
done
clear
View Answer play_arrow
question_answer 113) Yellowish edges appear in leaves deficient in
A)
\[C{{a}^{++}}\]
done
clear
B)
\[M{{g}^{++}}\]
done
clear
C)
\[{{K}^{+}}\]
done
clear
D)
Sulphur
done
clear
View Answer play_arrow
question_answer 114) Which one is responsible for opening and closing of stomata ?
A)
Rise in pH of guard cell causes hydrolysis of starch
done
clear
B)
Cytokinins and cAMP are requireds
done
clear
C)
Abscisic acid promotes closure
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 115) Connecting link between Echinodermata and Chordata is
A)
Peripatus
done
clear
B)
Archaeopteryx
done
clear
C)
Balanoglossus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 116) Scientists pin pointed the location of colour processing centres in human visual cortex by means of
A)
PET scanning
done
clear
B)
NMR scanning
done
clear
C)
CT scanning
done
clear
D)
Ultra sound imaging
done
clear
View Answer play_arrow
question_answer 117) AZT is the treatment of
A)
Malaria
done
clear
B)
AIDS
done
clear
C)
TB
done
clear
D)
Kala-azar
done
clear
View Answer play_arrow
question_answer 118) What is interferon ?
A)
Secretion in response to viral infection by the cell
done
clear
B)
Secretion in response to bacterial infection
done
clear
C)
Secretion in response to fungi infection
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 119) Industrial production of ethanol from starch is brought about by certain species of
A)
Pencillium
done
clear
B)
Azotobacter
done
clear
C)
Saccharomyces
done
clear
D)
Lactobacillus
done
clear
View Answer play_arrow
question_answer 120) The stimulant present in tea coca and coffee is
A)
cocaine
done
clear
B)
tannin
done
clear
C)
astringent
done
clear
D)
caffeine
done
clear
View Answer play_arrow
question_answer 121) Which is true ?
A)
Umbel is racemose inflorescence where- stalked flower aggregate on a flat receptacle
done
clear
B)
In raceme the main axis is shortened and flowers are borne acropetally
done
clear
C)
Spadix is a pendulous spike with main axis much flattened
done
clear
D)
Spike is a racemose inflorescence having sessile flowers
done
clear
View Answer play_arrow
question_answer 122) The chief anatomical feature of the family Cruciferae is the presence of
A)
latex
done
clear
B)
pectin
done
clear
C)
alkaloides
done
clear
D)
myrosin
done
clear
View Answer play_arrow
question_answer 123) Winged fruits are found is
A)
Moringa
done
clear
B)
Pinus
done
clear
C)
Dioscorea
done
clear
D)
Argemone
done
clear
View Answer play_arrow
question_answer 124) Inflorescence is edible in Brassica oleracea
A)
var. botrytes
done
clear
B)
var. capitata
done
clear
C)
var. gangyloides
done
clear
D)
var. germifera
done
clear
View Answer play_arrow
question_answer 125) Alternate phyllotaxy in which the sixth leaf lies above the first leaf after completing two circles is known as
A)
distichous
done
clear
B)
tristichous
done
clear
C)
pentastichous
done
clear
D)
octastichous
done
clear
View Answer play_arrow
question_answer 126) Nephridia of earthworm are analogous to
A)
nematoblasts of Hydra
done
clear
B)
tracheae of insects
done
clear
C)
flame cells of Planaria
done
clear
D)
gills of prawn
done
clear
View Answer play_arrow
question_answer 127) Cockroach blood does not contain respiratory pigment. It means
A)
it does not respire
done
clear
B)
cockroach respire anaerobically
done
clear
C)
oxygen passes to all the tissue through diffusion
done
clear
D)
oxygen reaches tissue through tracheoles
done
clear
View Answer play_arrow
question_answer 128) Frog is
A)
ammonolitic
done
clear
B)
ureotelic
done
clear
C)
uricotelic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 129) Structure present in man but absent in frog
A)
salivary gland
done
clear
B)
pancreas
done
clear
C)
adrenal gland
done
clear
D)
thyroid gland
done
clear
View Answer play_arrow
question_answer 130) Respiration without distinct respiratory oxgan occur in
A)
cockroach
done
clear
B)
frog
done
clear
C)
earthworm
done
clear
D)
fish
done
clear
View Answer play_arrow
question_answer 131) Aristotles lantern is connected with
A)
respiration
done
clear
B)
mastication
done
clear
C)
excretion
done
clear
D)
support
done
clear
View Answer play_arrow
question_answer 132) Brunners glands occur in
A)
sub-mucosa of duodenum
done
clear
B)
sub-mucosa of stomach
done
clear
C)
mucosa of oesophagus
done
clear
D)
mucosa of ileum
done
clear
View Answer play_arrow
question_answer 133) Vitamin needed for blood coagulation is
A)
E
done
clear
B)
D
done
clear
C)
K
done
clear
D)
C
done
clear
View Answer play_arrow
question_answer 134) During expiration diaphragm becomes
A)
flattened
done
clear
B)
dome shaped
done
clear
C)
oblique
done
clear
D)
normal
done
clear
View Answer play_arrow
question_answer 135) \[C{{O}_{2}}\] is carried in blood as
A)
sodium bicarbonate
done
clear
B)
sodium carbonate
done
clear
C)
potassium carbonate
done
clear
D)
magnesium carbonate
done
clear
View Answer play_arrow
question_answer 136) Pace maker of heart is
A)
AV node
done
clear
B)
Bundle of His
done
clear
C)
SA node
done
clear
D)
Purkinje fibre
done
clear
View Answer play_arrow
question_answer 137) Alary muscles of cockroach are associated with
A)
Malpighian tubules and excretion
done
clear
B)
trachea and respiration
done
clear
C)
heart and circulation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 138) Uric acid gets deposited in small joints to produce
A)
rheumatid arthritis
done
clear
B)
gout
done
clear
C)
osteoarthritis
done
clear
D)
bursitis
done
clear
View Answer play_arrow
question_answer 139) Number of vertebrae in human skeleton is
A)
30
done
clear
B)
32
done
clear
C)
33
done
clear
D)
35
done
clear
View Answer play_arrow
question_answer 140) Pelvic girdle consist of
A)
ilium
done
clear
B)
ilium, ischium and pubis
done
clear
C)
ilium and ischium
done
clear
D)
ischium and pubis
done
clear
View Answer play_arrow
question_answer 141) Foramen of Monro is an aperture between
A)
third and 4th ventricles
done
clear
B)
rhinocoel and diacoel
done
clear
C)
lateral and third ventricle
done
clear
D)
diacoel and metacoel
done
clear
View Answer play_arrow
question_answer 142) The sequence of ear ossicles from outside (tympanum) to inside is
A)
stapes, incus and malleus
done
clear
B)
malleus, incus and stapes
done
clear
C)
stapes, malleus and incus
done
clear
D)
incus, malleus and stapes
done
clear
View Answer play_arrow
question_answer 143) Organ of Golgi is the sensing structure formed at the junction of
A)
two nerves
done
clear
B)
two bones
done
clear
C)
nerve and muscles
done
clear
D)
muscles and tendon
done
clear
View Answer play_arrow
question_answer 144) Which is aminated hormone?
A)
Progesterone
done
clear
B)
Epinephrine
done
clear
C)
Estrogen
done
clear
D)
Relaxin
done
clear
View Answer play_arrow
question_answer 145) Ancestors of mammals belongs to
A)
Uherapsida
done
clear
B)
Omithischia
done
clear
C)
Silusoidea
done
clear
D)
Chelonea
done
clear
View Answer play_arrow
question_answer 146) Besides bats, echolocation also occurs in
A)
primates
done
clear
B)
wild cat
done
clear
C)
whales and dolphins
done
clear
D)
beavers
done
clear
View Answer play_arrow
question_answer 147) Kaziranga national park is famous for
A)
one homed rhinocerose
done
clear
B)
tiger leopard
done
clear
C)
nilgai and samber
done
clear
D)
sandpiper
done
clear
View Answer play_arrow
question_answer 148) Linkage map of X-chromosome of fruit fly has 66 map units with yellow body gene (y) at one end and bobbed hair (b) at the other end. The recombination frequency between y and b gene would be
A)
66%
done
clear
B)
\[\le 50%\]
done
clear
C)
>50%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 149) A living fossil is
A)
coelacanth
done
clear
B)
sphenodon
done
clear
C)
limulus
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 150) Which of the following is not functionally analogous with other in the group?
A)
Anthridium
done
clear
B)
Archegonium
done
clear
C)
Oogonium
done
clear
D)
Ovule
done
clear
View Answer play_arrow
question_answer 151) Appearance of teeth in the embryos of birds is an example of
A)
vestigial organs
done
clear
B)
ontogeny repeats phylogeny
done
clear
C)
Atavism
done
clear
D)
speciation
done
clear
View Answer play_arrow
question_answer 152) Which is the most recent in human evolution?
A)
Mesolithic
done
clear
B)
Upper Palaeolithic
done
clear
C)
Neolithic
done
clear
D)
Middle Palaeolithic
done
clear
View Answer play_arrow
question_answer 153) An enzyme carrier protein associated with cell membrane
A)
lipase
done
clear
B)
amylase
done
clear
C)
permease
done
clear
D)
catalase
done
clear
View Answer play_arrow
question_answer 154) Ultraphagocytosis (colloidopexy) is intake by cell of
A)
chromagen particles
done
clear
B)
small colloidal particles
done
clear
C)
large colloidal particles
done
clear
D)
it is very quick phagocytosis
done
clear
View Answer play_arrow
question_answer 155) Meiosis was discovered by
A)
Strasburger
done
clear
B)
Hofmerster
done
clear
C)
Sutton
done
clear
D)
Van Beneden
done
clear
View Answer play_arrow
question_answer 156) Plastids of an etiolated plant possess
A)
Phycobilins
done
clear
B)
carotenoids and xanthophylls
done
clear
C)
chlorophylloid and carotenoids
done
clear
D)
chlorophyll and carotenes
done
clear
View Answer play_arrow
question_answer 157) In an ecosystem, bacteria are considered as
A)
micro-consumers
done
clear
B)
macro-consumers
done
clear
C)
primary consumers
done
clear
D)
secondary consumers
done
clear
View Answer play_arrow
question_answer 158) Wilting occurs when there is
A)
transpiration higher than absorption
done
clear
B)
absorption higher than transpiration
done
clear
C)
higher relative humidity of atmosphere
done
clear
D)
excess root pressure
done
clear
View Answer play_arrow
question_answer 159) Hydroponics is growing plants in
A)
water
done
clear
B)
soil culture
done
clear
C)
solution of mineral nutrients
done
clear
D)
tissue culture medium
done
clear
View Answer play_arrow
question_answer 160) The process of photophosphorylation was discovered by
A)
Calvin
done
clear
B)
Arnon
done
clear
C)
Priestley
done
clear
D)
Warburg
done
clear
View Answer play_arrow
question_answer 161) First reaction in photosynthesis is
A)
photolysis of water
done
clear
B)
excitation of chlorophyll molecules
done
clear
C)
formation of ATP
done
clear
D)
fixation of \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 162) Oxidation of pyruvate to \[C{{O}_{2}}\] and \[{{H}_{2}}O\] occur through
A)
citric acid cycle
done
clear
B)
tricarboxylic acid
done
clear
C)
Krebs cycle
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 163) High milk yielding crossbreed Frieswal cow is product of
A)
Brown Swiss and Sahiwal
done
clear
B)
Friesian and Sahiwal
done
clear
C)
Holstein and Tharpaikar
done
clear
D)
Brown Swiss and Red Sindhi
done
clear
View Answer play_arrow
question_answer 164) A plant having nitrogen fixing bacteria is
A)
cotton
done
clear
B)
wheat
done
clear
C)
gram
done
clear
D)
mustard
done
clear
View Answer play_arrow
question_answer 165) Which of the following is correct for LSD morphine and charas respectively?
A)
Claviceps, Papaver somniferum, Cannabis
done
clear
B)
Claviceps, Cannabis, Papaver somniferum
done
clear
C)
Claviceps, Cannabis, Rauwolffia
done
clear
D)
Claviceps, Fusarium, Cannabis
done
clear
View Answer play_arrow
question_answer 166) Biopesticides do not control
A)
viruses
done
clear
B)
nematodes
done
clear
C)
Both (a) and (b)
done
clear
D)
fungi and bacteria
done
clear
View Answer play_arrow
question_answer 167) What is true of plasmids ?
A)
They are found in viruses
done
clear
B)
They are main parts of chromosomes
done
clear
C)
They are widely used in gene transfer
done
clear
D)
They contain genes for vital activities
done
clear
View Answer play_arrow
question_answer 168) The type of immunoglobulin present in the colostrum secreted by mammary glands is
A)
IgD
done
clear
B)
IgE
done
clear
C)
IgG
done
clear
D)
IgA
done
clear
View Answer play_arrow
question_answer 169) Cornea transplantation is quite common because
A)
it is easily available
done
clear
B)
it is easily transplanted
done
clear
C)
it does not invite immune response due to absence of blood
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 170) Genes involved in cancer are
A)
cancer gene
done
clear
B)
oncogenes
done
clear
C)
tumour genes
done
clear
D)
regulater genes
done
clear
View Answer play_arrow
question_answer 171) The aleurone layer in maize grain is especially rich in
A)
lipids
done
clear
B)
auxin
done
clear
C)
protein
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 172) Dispersal of fruits in opium (poppy) occurs through shaking by wind by
A)
explosive mechanism
done
clear
B)
parachute mechanism
done
clear
C)
cencer mechanism
done
clear
D)
jacular mechanism
done
clear
View Answer play_arrow
question_answer 173) Sepals (calyx) are modified into wings in the fruits called samaroid. One such example is
A)
Shorea (sal)
done
clear
B)
Dodonaea
done
clear
C)
Acer
done
clear
D)
Morenga
done
clear
View Answer play_arrow
question_answer 174) Ovary in Solanaceae is
A)
bicarpellary, syncarpous, superior
done
clear
B)
monocarpellary, syncarpous, superior
done
clear
C)
tricarpellary, syncarpous, superior
done
clear
D)
multicarpellary, syncarpous, superior
done
clear
View Answer play_arrow
question_answer 175) Elongated peduncle bearing pedicellate flower continuously in acropetal order is
A)
corymb
done
clear
B)
umbel
done
clear
C)
raceme
done
clear
D)
head
done
clear
View Answer play_arrow
question_answer 176) In Pheretima, septa are absent between segments
A)
5/6 and 10 and 11
done
clear
B)
absent in first four
done
clear
C)
5/6 and 7/8
done
clear
D)
7/8 and 6/7
done
clear
View Answer play_arrow
question_answer 177) Peritrophic membrane is associated with a part of digestive system in
A)
Ascaris
done
clear
B)
Neries
done
clear
C)
Antedon
done
clear
D)
Periplanata
done
clear
View Answer play_arrow
question_answer 178) Abdomen of cockroach has segments
A)
6
done
clear
B)
10
done
clear
C)
11
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 179) In frog, two phalanges occur in
A)
pollex
done
clear
B)
hallux
done
clear
C)
third finger
done
clear
D)
third toe
done
clear
View Answer play_arrow
question_answer 180) In frog jelly around the eggs is deposited
A)
in water after fertilization
done
clear
B)
in water during fertilization
done
clear
C)
in the oviduct
done
clear
D)
in the ovary
done
clear
View Answer play_arrow
question_answer 181) The hormone secretin is produced by
A)
pancreas and influence the conversion of glycogen to glucose
done
clear
B)
adrenal gland and accelerate heart beat
done
clear
C)
testis and produces male secondary sex character
done
clear
D)
small intestine and stimulate pancreas
done
clear
View Answer play_arrow
question_answer 182) Triple "F" or gland for flight, fight and fright/lifesaving/emergency gland is
A)
thyroid
done
clear
B)
thymus
done
clear
C)
pituitary
done
clear
D)
adrenal
done
clear
View Answer play_arrow
question_answer 183) A serious eye defects which can lead to blindness is
A)
myopia
done
clear
B)
hyper metropia
done
clear
C)
presbyopia
done
clear
D)
glaucoma
done
clear
View Answer play_arrow
question_answer 184) Cranial nerve showing maximum branching is
A)
trigeminal
done
clear
B)
vagus
done
clear
C)
optic
done
clear
D)
facial
done
clear
View Answer play_arrow
question_answer 185) Which one is the spinal nerve ?
A)
Trigeminal
done
clear
B)
Hypoglossal
done
clear
C)
Olfactory
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 186) Haversian system is diagnostic feature of
A)
avian bones
done
clear
B)
reptilian bones
done
clear
C)
mammalian bones
done
clear
D)
bone of all animals
done
clear
View Answer play_arrow
question_answer 187) Hinge joint occur between
A)
humerus and radio-ulna
done
clear
B)
femur and pelvic girdle
done
clear
C)
hamerus and pectoral girdle
done
clear
D)
skull and atlas
done
clear
View Answer play_arrow
question_answer 188) ADH controls water permeability of
A)
collecting tube
done
clear
B)
proximal convoluted tube
done
clear
C)
distal convoluted tubule
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 189) Rise in heart beat, cardiac output, blood pressure and blood sugar occurs during emergency by hormone
A)
Aldosterone
done
clear
B)
antidiuretic hormone
done
clear
C)
Norepinephrine
done
clear
D)
oxytocin
done
clear
View Answer play_arrow
question_answer 190) Oxyhaemoglobin dissociates into oxygen and deoxyhaemoglobin at
A)
low \[{{O}_{2}}\] pressure in tissue
done
clear
B)
high \[{{O}_{2}}\] pressure in tissue
done
clear
C)
equal \[{{O}_{2}}\] pressure inside and outside tissue
done
clear
D)
all times irrespective of \[{{O}_{2}}\] pressure
done
clear
View Answer play_arrow
question_answer 191) Hydrolytic enzyme which acts at low pH is
A)
\[\alpha \]-amylase
done
clear
B)
protease
done
clear
C)
hydrolases
done
clear
D)
peroxidases
done
clear
View Answer play_arrow
question_answer 192) Ornithine cycle is related to
A)
respiration
done
clear
B)
excretion
done
clear
C)
digestion
done
clear
D)
nutrition
done
clear
View Answer play_arrow
question_answer 193) Sericteris (silk gland) are modified
A)
intestinal gland
done
clear
B)
gastric gland
done
clear
C)
salivary gland
done
clear
D)
endocrine glands
done
clear
View Answer play_arrow
question_answer 194) Which one is not a larval stage of flatwarm ?
A)
Redia
done
clear
B)
Cercaria
done
clear
C)
Bipinnaria
done
clear
D)
Miracedium
done
clear
View Answer play_arrow
question_answer 195) Ascaris larva is called
A)
cysticerus
done
clear
B)
rhabditiform
done
clear
C)
hexacanth
done
clear
D)
onchosphere
done
clear
View Answer play_arrow
question_answer 196) Assertion: Prokaryotic genome consis of a single circular DNA molecule. Reason: Genetic variations do not occurs in prokaryotes.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 197) Assertion: Enzymes are proteins which catalyse biochemical reactions. Reason: The enzymes itself unchanged in the reaction, its presence allows the reaction to take place.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 198) Assertion: DNA code is copied in the synthesis of mRNA. Reason: tRNA moves out of nucleus and after attaching on ribosomes form the template.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 199) Assertion: Auxin helps in apical dominance. Reason: If a pest of auxin is painted on the cut end of the decapitated shoot. The lateral buads remain inhibited.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 200) Assertion: Fatty liver syndrome occurs by Reason: Alcoholics have a rate of carcinoma 10 times higher than that expected in the general population.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow