A) \[1.8atm\]
B) \[3atm\]
C) \[0.3atm\]
D) \[0.18atm\]
Correct Answer: A
Solution :
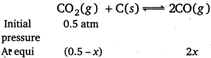 \[\therefore \] \[(0.5-x)+2x=0.8\] \[x=0.3atm.\] \[\therefore \] \[{{p}_{C{{O}_{2}}}}=0.5-0.3=0.2\] \[{{p}_{CO}}=2x=2\times 0.3=0.6atm\] \[K=\frac{p_{CO}^{2}}{{{p}_{C{{O}_{2}}}}}=\frac{{{(0.6)}^{2}}}{0.2}=1.8atm\]
\[\therefore \] \[(0.5-x)+2x=0.8\] \[x=0.3atm.\] \[\therefore \] \[{{p}_{C{{O}_{2}}}}=0.5-0.3=0.2\] \[{{p}_{CO}}=2x=2\times 0.3=0.6atm\] \[K=\frac{p_{CO}^{2}}{{{p}_{C{{O}_{2}}}}}=\frac{{{(0.6)}^{2}}}{0.2}=1.8atm\]
You need to login to perform this action.
You will be redirected in
3 sec
