A) \[4.5\]
B) \[2.3\]
C) \[3.8\]
D) \[4\]
Correct Answer: A
Solution :
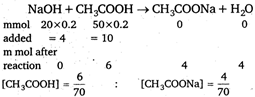 \[pH=-\log (1.8\times {{10}^{-5}})+\log \frac{4/70}{6/70}\] \[=4.56\]
\[pH=-\log (1.8\times {{10}^{-5}})+\log \frac{4/70}{6/70}\] \[=4.56\]
You need to login to perform this action.
You will be redirected in
3 sec
