A) \[[Ni{{(N{{H}_{3}})}_{6}}]C{{l}_{2}}\]
B) \[N{{a}_{3}}[Fe{{F}_{6}}]\]
C) \[[Cr{{({{H}_{2}}O)}_{6}}]S{{O}_{4}}\]
D) \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
Correct Answer: D
Solution :
Ferrocyanide ion \[{{[Fe{{(CN)}_{6}}]}^{4-}}\] is diamagnetic in nature hence \[{{K}_{4}}[Fe{{(CN)}_{6}}]\] complex has zero magnetic moment.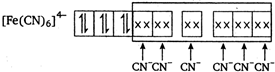
You need to login to perform this action.
You will be redirected in
3 sec
