Answer:
X = 20; 2, 8, 8, 2
Y = 17; 2, 8, 7
\[\underset{2,8,8,2}{\mathop{X}}\,\to {{X}^{2+}}+2{{e}^{-}}\]
\[2Y+2{{e}^{-}}\to 2{{Y}^{-}}\]
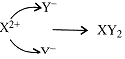
Electron-dot structure:
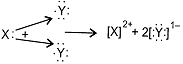
The nature of bond will be ionic.
You need to login to perform this action.
You will be redirected in
3 sec
