| Name the substance oxidised and the substance reduced, and also identify the oxidising agent and reducing agents in the following reaction: |
| (a) \[\mathbf{3Mn}{{\mathbf{O}}_{\mathbf{2}}}\mathbf{+4Al}\to \mathbf{3Mn+2A}{{\mathbf{l}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}\] |
| (b) \[\mathbf{F}{{\mathbf{e}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}\mathbf{+3CO}\to \mathbf{2Fe+3C}{{\mathbf{O}}_{\mathbf{2}}}\] |
| (c) \[\mathbf{S}{{\mathbf{O}}_{\mathbf{2}}}\mathbf{+2}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{S}\to \mathbf{3S+2}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\] |
Answer:
(a) 
Substance oxidised \[=Al\] Substance reduced \[=Mn{{O}_{2}}\] Oxidising agent \[=Mn{{O}_{2}}\] Reducing agent \[=Al\] (b) 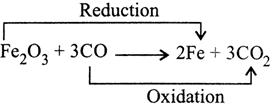
Substance oxidised \[=CO\] Substance reduced \[=F{{e}_{2}}{{O}_{3}}\] Oxidising agent \[=F{{e}_{2}}{{O}_{3}}\] Reducing agent \[=CO\] (c) 
Substance oxidised \[={{H}_{2}}S\] Substance reduced \[=S{{O}_{2}}\] Oxidising agent \[=S{{O}_{2}}\] Reducing agent \[={{H}_{2}}S\]
You need to login to perform this action.
You will be redirected in
3 sec
