question_answer 1) A block of metal weighing 2 kg is resting on a frictionless plane. It is strucked by a jet releasing. Water at a rate of 1 kg/s and at a speed of 5 m/s. The initial acceleration of the block will be
A)
\[2.5\,m/{{s}^{2}}\]
done
clear
B)
\[5.0\,m/{{s}^{2}}\]
done
clear
C)
\[10\,m/{{s}^{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 2) A body of mass 2 kg is being dragged with uniform velocity of 2 m/s on a rough horizontal plane. The coefficient of friction between the body and the surface is 0.20. The amount of heat generated in 5 s is V = 4.2 J/cal and \[g=9.8\,m/{{s}^{2}}\])
A)
9.33 cal
done
clear
B)
10.21 cal
done
clear
C)
12.67 cal
done
clear
D)
13.34 cal
done
clear
View Answer play_arrow
question_answer 3) From an automatic gun a man fires 360 bullet per minute with a speed of 360 km/h. If each weighs 20g, the power of the gun is
A)
600 W
done
clear
B)
300 W
done
clear
C)
150 W
done
clear
D)
75 W
done
clear
View Answer play_arrow
question_answer 4) An annular ring with inner and outer radii \[{{R}_{1}}\] and \[{{R}_{2}}\]is rolling without slippling with a uniform angular speed. The ratio of the forces experienced by the two particles situated on the inner and outer parts of the ring, \[\frac{{{F}_{1}}}{{{F}_{2}}}\]is
A)
1
done
clear
B)
\[\left( \frac{{{R}_{1}}}{{{R}_{2}}} \right)\]
done
clear
C)
\[\frac{{{R}_{2}}}{{{R}_{1}}}\]
done
clear
D)
\[{{\left( \frac{{{R}_{1}}}{{{R}_{2}}} \right)}^{2}}\]
done
clear
View Answer play_arrow
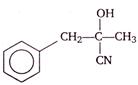
question_answer 5) A rubber cord catapult has cross-sectional area \[25\,m{{m}^{2}}\] and initial length of rubber cord is 10 cm. It is stretched to 5 cm and then released to project a missile of mass 5 g. Taking \[{{Y}_{rubber}}\,=5\times {{10}^{8}}\,N/{{m}^{2}}\]. Velocity of projected missile is
A)
\[20\,m/{{s}^{-1}}\]
done
clear
B)
\[100\,m/{{s}^{-1}}\]
done
clear
C)
\[250\,m/{{s}^{-1}}\]
done
clear
D)
\[200\,m/{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 6) If the work done in blowing a bubble of volume V is W then the work done in blowing bubble of volume 2 V from the same soap solution will be
A)
W/2
done
clear
B)
\[\sqrt{\text{2}}\,\text{W}\]
done
clear
C)
\[\sqrt[\text{3}]{\text{2}}\,\text{W}\]
done
clear
D)
\[\sqrt[\text{3}]{\text{4}}\,\text{W}\]
done
clear
View Answer play_arrow
question_answer 7) 540 g of ice of \[0{}^\circ C\] is mixed with 540 g of water at \[80{}^\circ C\]. The final temperature of the mixture is
A)
\[0{}^\circ C\]
done
clear
B)
\[40{}^\circ C\]
done
clear
C)
\[80{}^\circ C\]
done
clear
D)
less than \[0{}^\circ C\]
done
clear
View Answer play_arrow
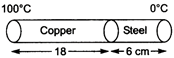
question_answer 8)
The coefficient of thermal conductivity of copper is 9 times that of steel. In the composite cylindrical bar shown in the figure, what will be the temperature at the junction of copper and steel?
A)
\[75{}^\circ C\]
done
clear
B)
\[67{}^\circ C\]
done
clear
C)
\[33{}^\circ C\]
done
clear
D)
\[25{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 9) In order to double the frequency of the fundamental note emitted by a stretched string, the length is reduced to\[\frac{\text{3}}{\text{4}}\text{th}\] of the original length and the tension is changed. The factor by which the tension is to be changed is
A)
\[\frac{\text{3}}{8}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[\frac{8}{9}\]
done
clear
D)
\[\frac{9}{4}\]
done
clear
View Answer play_arrow
question_answer 10) A \[\text{10}\,\text{F}\] capacitor is charged to a potential difference of 1000 V. The terminals of the charged capacitor are disconnected from the power supply and connected to the terminals of an uncharged 6uF capacitor. What is the final potential difference across each capacitor?
A)
167 V
done
clear
B)
100 V
done
clear
C)
625 V
done
clear
D)
250 V
done
clear
View Answer play_arrow
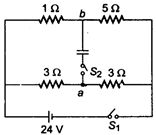
question_answer 11)
In the circuit shown in figure, switch S; is initially closed and \[{{S}_{2}}\] is open. Find \[{{V}_{a}}-{{V}_{b.}}\].
A)
4 V
done
clear
B)
8 V
done
clear
C)
12 V
done
clear
D)
16 V
done
clear
View Answer play_arrow
question_answer 12) Two bulbs consume same power when operated at 200V and 300V respectively. When these bulbs are connected in series across a DC source of 500 V, then
A)
ratio of potential differences across them is 3/2
done
clear
B)
ratio of potential differences across them is 9/4
done
clear
C)
ratio of powers consumed across them is 4/9
done
clear
D)
ratio of powers consumed across them is 2/3
done
clear
View Answer play_arrow
question_answer 13) Two magnets are held together in a vibration magnetometer and are allowed to oscillate in the earths magnetic field with like poles together, 12 oscillations per minute are made but for unlike poles together only 4 oscillations per minute are executed. The ratio of their magnetic moments is
A)
3 : 1
done
clear
B)
1 : 3
done
clear
C)
3 : 5
done
clear
D)
5 : 4
done
clear
View Answer play_arrow
question_answer 14) The molar heat capacity in a process of a diatomic gas if it does a work of \[\frac{Q}{4},\]when a heat of Q is supplied to it is
A)
\[\frac{2}{5}R\]
done
clear
B)
\[\frac{5}{2}R\]
done
clear
C)
\[\frac{10}{3}R\]
done
clear
D)
\[\frac{6}{7}R\]
done
clear
View Answer play_arrow
question_answer 15) The dimensions of \[\frac{a}{b}\]in the equation\[P=\frac{a-{{t}^{2}}}{bx},\] where p is pressure, x is distance and t is time, are
A)
\[[M{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{2}}{{T}^{-3}}]\]
done
clear
C)
\[[M{{L}^{3}}{{T}^{-1}}]\]
done
clear
D)
\[[L{{T}^{-3}}]\]
done
clear
View Answer play_arrow
question_answer 16) Dimensions of \[\frac{1}{{{\mu }_{0}}{{\varepsilon }_{0}}},\]where symbols have their usual meanings are
A)
\[[L{{T}^{-1}}]\]
done
clear
B)
\[[{{L}^{-1}}T]\]
done
clear
C)
\[[{{L}^{-}}^{2}{{T}^{2}}]\]
done
clear
D)
\[[{{L}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 17) A body is projected vertically up with a velocity v and after some time it returns to the point from which it was projected. The average velocity and average speed of the body for the total time of flight are
A)
\[\frac{{\mathbf{\vec{v}}}}{2}and\frac{v}{2}\]
done
clear
B)
\[0\,and\frac{v}{2}\]
done
clear
C)
0 and 0
done
clear
D)
\[\frac{{\mathbf{\vec{v}}}}{2}and\,0\]
done
clear
View Answer play_arrow
question_answer 18) A sphere of mass m is tied to end of a string of length\[l\]and rotated through the other end along a horizontal circular path with speed v. The work done in full horizontal circle is
A)
zero
done
clear
B)
\[\left( \frac{m{{v}^{2}}}{l} \right)2\pi l\]
done
clear
C)
\[mg.\,2\pi l\]
done
clear
D)
\[\left( \frac{m{{v}^{2}}}{l} \right)(l)\]
done
clear
View Answer play_arrow
question_answer 19) For a body moving in a circular path, a condition for no skidding if\[\mu \] is the coefficient of friction, is
A)
\[\frac{m{{v}^{2}}}{r}\le \mu mg\]
done
clear
B)
\[\frac{m{{v}^{2}}}{r}\ge \mu mg\]
done
clear
C)
\[\frac{v}{r}=\mu g\]
done
clear
D)
\[\frac{m{{v}^{2}}}{r}=\mu mg\]
done
clear
View Answer play_arrow
question_answer 20) A car is moving with a uniform speed on a level road. Inside the car there is a balloon filled with helium and attached to a piece of string tied to the floor. The string is observed to be vertical. The car now takes a left turn maintaining the speed on the level road. The balloon in the car will
A)
continue to remain vertical
done
clear
B)
burst while taking the curve
done
clear
C)
be thrown to the right side
done
clear
D)
be thrown to the left side
done
clear
View Answer play_arrow
question_answer 21) Two balls of masses \[{{m}_{1}}\] and\[{{m}_{2}}\] are separated from each other by a powder charge placed between them. The whole system is at rest on the ground. Suddenly the powder charge explodes and masses are pushed apart. The mass\[{{m}_{1}}\]travels a distance \[{{S}_{1}}\] and stops. If the coefficients of friction between the balls and ground are same, the mass \[{{m}_{2}}\]stops after travelling the distance
A)
\[{{s}_{2}}=\frac{{{m}_{1}}}{{{m}_{2}}}{{s}_{1}}\]
done
clear
B)
\[{{s}_{2}}=\frac{{{m}_{2}}}{{{m}_{1}}}{{s}_{1}}\]
done
clear
C)
\[{{s}_{2}}=\frac{{{m}_{1}}}{m_{2}^{2}}{{s}_{1}}\]
done
clear
D)
\[{{s}_{2}}=\frac{m_{2}^{1}}{m_{1}^{2}}{{s}_{1}}\]
done
clear
View Answer play_arrow
question_answer 22) A cricket ball of mass 250 g collides with a bat with velocity 10 m/s and returns with the same velocity within 0.01 s. The force acted on bat is
A)
25 N
done
clear
B)
50 N
done
clear
C)
250 N
done
clear
D)
500 N
done
clear
View Answer play_arrow
question_answer 23) When a spring is stretched by 2 cm, it stores 100 J of energy. If it is stretched further by 2 cm, the stored energy will be increased by
A)
100 J
done
clear
B)
200 J
done
clear
C)
300 J
done
clear
D)
400 J
done
clear
View Answer play_arrow
question_answer 24) The kinetic energy of a body decreases by 36%. The decrease in the momentum is
A)
36%
done
clear
B)
20%
done
clear
C)
8%
done
clear
D)
6%
done
clear
View Answer play_arrow
question_answer 25) A wheel has a speed of 1200 revolutions per minute and is made to slow down at a rate of 4 radians/s2.The number of revolutions it makes before coming to rest is
A)
143
done
clear
B)
272
done
clear
C)
314
done
clear
D)
722
done
clear
View Answer play_arrow
question_answer 26) When a uniform solid sphere and a disc of the same mass and of the same radius rolls down an inclined smooth plane from rest to the same distance, then the ratio of the time taken by them is
A)
15 : 14
done
clear
B)
\[{{15}^{2}}:{{14}^{2}}\]
done
clear
C)
\[\sqrt{14}:\sqrt{15}\]
done
clear
D)
14 : 15
done
clear
View Answer play_arrow
question_answer 27) The diameters of two planets are in the ratio 4 : 1 and their mean densities in the ratio 1 : 2. The acceleration due to gravity on the planets will be in ratio
A)
1 : 2
done
clear
B)
2 : 3
done
clear
C)
2 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 28) At the surface of a certain planet, acceleration due to gravity is one-quarter of that on earth. If a brass ball is transported to this planet, then which one of the following statements is not correct?
A)
The mass of the brass ball on this planet is a quarter of its mass as measured on earth
done
clear
B)
The weight of the brass ball on this planet is a quarter of the weight as measured on earth
done
clear
C)
The brass ball has the same mass on the planet as on earth
done
clear
D)
The brass ball has the same volume on the other planet as on earth
done
clear
View Answer play_arrow
question_answer 29) A clock S is based on oscillation of a spring and a clock P based on pendulum motion. Both clocks run at the same rate on earth. On a planet having the same density as earth but twice the radius
A)
S will run faster than P
done
clear
B)
P will run faster than S
done
clear
C)
they will both run at the same rate as on the earth
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 30) At a given place where acceleration due to gravity is \[g\,m/{{s}^{2}},\] a sphere of lead of density \[d\,kg/{{m}^{3}}\] is getting released in a column of liquid of density\[\text{ }\!\!\!\!\text{ }\text{ }\!\!\!\!\text{ }\] \[kg\,/{{m}^{3}}\]. If \[d>\rho ,\] the sphere will
A)
fall vertically with an acceleration \[g\,m/{{s}^{2}}\]
done
clear
B)
fall vertically with no acceleration
done
clear
C)
fall vertically with an acceleration\[\left( \frac{d-\rho }{d} \right)\]
done
clear
D)
fall vertically with an acceleration\[\left( \frac{\rho }{d} \right)\]
done
clear
View Answer play_arrow
question_answer 31) The diameter of a brass rod is 4 mm and Youngs modulus of brass is \[9\times {{10}^{10}}\,N/{{m}^{2}}\]. The force required to stretch by 0.1% of its length is
A)
\[360\,\pi N\]
done
clear
B)
36 N
done
clear
C)
\[144\pi \times {{10}^{3}}N\]
done
clear
D)
\[36\pi \times {{10}^{5}}N\]
done
clear
View Answer play_arrow
question_answer 32) A ball falling in a lake of depth 200m shows 0.1% decrease in its volume at the bottom. What is the bulk modulus of the material of the ball
A)
\[19.6\,\times {{10}^{8}}\,N/{{m}^{2}}\]
done
clear
B)
\[19.6\,\times {{10}^{-10}}\,N/{{m}^{2}}\]
done
clear
C)
\[19.6\,\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
D)
\[19.6\,\times {{10}^{-8}}\,N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 33) If one end of a wire is fixed with a rigid support and the other end is stretched by a force of 10 N, then the increase in length is 0.5 mm. The ratio of the energy of the wire and the work done in displacing it through 1.5 mm by the weight is
A)
\[\frac{1}{3}\]
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 34) The increase in length on stretching a wire is 0.05%. If its Poissons ratio is 0.4, then its diameter will be
A)
reduced by 0.02%
done
clear
B)
reduced by 0.1%
done
clear
C)
increased by 0.02%
done
clear
D)
decreased by 0.4%
done
clear
View Answer play_arrow
question_answer 35) Work done in splitting a drop of water of 1 mm radius into 10 droplets is (surface tension of water \[=72\,\times {{10}^{-3}}J/{{m}^{2}}\])
A)
\[9.58\,\times {{10}^{-5}}J\]
done
clear
B)
\[8.95\,\times {{10}^{-5}}J\]
done
clear
C)
\[5.89\,\times {{10}^{-5}}J\]
done
clear
D)
\[5.98\,\times {{10}^{-6}}J\]
done
clear
View Answer play_arrow
question_answer 36) Water rises to a height of 16.3 cm in a capillary tube of height 18 cm above the water level. If the tube is cut at a height of 12 cm, then
A)
water will come as a fountain from the capillary tube
done
clear
B)
water will stay at a height of 12 cm in the capillary tube
done
clear
C)
the height of the water in the capillary tube will be 10.3 cm
done
clear
D)
water will flow down the sides of the capillary tube
done
clear
View Answer play_arrow
question_answer 37) Two bodies are in equilibrium when suspended in water from the arms of a balance. The mass of one body is 36 g and its density is \[9\,g/c{{m}^{3}}\]. If the mass of the other is 48 g, its density in \[g/c{{m}^{3}}\] is
A)
\[\frac{4}{3}\]
done
clear
B)
\[\frac{3}{2}\]
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 38) The fraction of a floating object of volume\[{{V}_{0}}\] and density\[{{d}_{0}}\]above the surface of liquid of density\[d\] will be
A)
\[\frac{{{d}_{0}}}{d}\]
done
clear
B)
\[\frac{d{{d}_{0}}}{d+{{d}_{0}}}\]
done
clear
C)
\[\frac{d-{{d}_{0}}}{d}\]
done
clear
D)
\[\frac{d{{d}_{0}}}{d-{{d}_{0}}}\]
done
clear
View Answer play_arrow
question_answer 39) A constant pressure air thermometer gave a reading of 47.5 units of volume when immersed in ice cold water, and 67 units in a boiling liquid. The boiling point of the liquid will be
A)
\[135{}^\circ C\]
done
clear
B)
\[125{}^\circ C\]
done
clear
C)
\[112{}^\circ C\]
done
clear
D)
\[100{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 40) A breaker is completely filled with water at \[4{}^\circ C\]. It will overflow if
A)
heated above \[4{}^\circ C\]
done
clear
B)
cooled below \[4{}^\circ C\]
done
clear
C)
both heated and cooled above and below \[4{}^\circ C\] respectively
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 41) The following sets of values for\[{{C}_{V}}\]and \[{{C}_{P}}\] of a gas has been reported by different students. The units are cal/g-mol-K. Which of these sets is most reliable?
A)
\[{{C}_{V}}=3,\,{{C}_{p}}=5\]
done
clear
B)
\[{{C}_{V}}=4,\,{{C}_{p}}=6\]
done
clear
C)
\[{{C}_{V}}=3,\,{{C}_{p}}=2\]
done
clear
D)
\[{{C}_{V}}=3,\,{{C}_{p}}=4.2\]
done
clear
View Answer play_arrow
question_answer 42) A cylinder of capacity 20 L is filled with H2 gas. The total average kinetic energy of translator motion of its molecules is \[1.5\,\times {{10}^{5}}J\]. The pressure of hydrogen in the cylinder is
A)
\[2\times {{10}^{6}}\,N/{{m}^{2}}\]
done
clear
B)
\[3\times {{10}^{6}}\,N/{{m}^{2}}\]
done
clear
C)
\[4\times {{10}^{6}}\,N/{{m}^{2}}\]
done
clear
D)
\[5\times {{10}^{6}}\,N/m\]
done
clear
View Answer play_arrow
question_answer 43) An engineer claims to have made an engine delivering 10 kW power with fuel consumption of 1 g/s. The calorific value of the fuel is 2 kcal/g. The claim of the engineer is
A)
valid
done
clear
B)
invalid
done
clear
C)
depends on engine design
done
clear
D)
depends of the load
done
clear
View Answer play_arrow
question_answer 44) Find the change in the entropy in the following process 100 gm of ice at \[0{}^\circ C\] melts when dropped in a bucket of water at \[50{}^\circ C\]. (Assume temperature of water does not change)
A)
- 4.5 cal/K
done
clear
B)
+ 4.5 cal/K
done
clear
C)
+ 5.4 cal/K
done
clear
D)
- 5.4 cal /K
done
clear
View Answer play_arrow
question_answer 45) A cylinder of fixed capacity 44.8 L contains a monoatomic gas at standard temperature and pressure. The amount of heat required to raise the temperature of cylinder by \[10{}^\circ C\] will be (R = universal gas constant)
A)
R
done
clear
B)
10 R
done
clear
C)
20 R
done
clear
D)
30 R
done
clear
View Answer play_arrow
question_answer 46) Two spheres of different materials one with double the radius and one-fourth with thickness of the other, are filled with ice. If the time taken for complete melting of ice in the large radius one is 25 min and that for smaller one is 16 min, the ratio of thermal conductivities of the materials of larger sphere to the smaller sphere is
A)
4 : 5
done
clear
B)
5 : 4
done
clear
C)
25 : 1
done
clear
D)
1 : 25
done
clear
View Answer play_arrow
question_answer 47) An ideal black body at room temperature is thrown into a furnace. It is observed that
A)
initially, it is the darkest body and at later times the brightest
done
clear
B)
it is the darkest body at all times
done
clear
C)
it cannot be distinguished at all times
done
clear
D)
initially, it is the darkest body and at later times it cannot be distinguished
done
clear
View Answer play_arrow
question_answer 48) An emf of 100 mV is induced in a coil when the current in another nearby coil becomes 10 A from zero in 0.1 s. The coefficient of mutual induction between the two coils will be
A)
1 mH
done
clear
B)
10 mH
done
clear
C)
100 mH
done
clear
D)
1000 mH
done
clear
View Answer play_arrow
question_answer 49) In an L-R circuit, time constant is that time in which current grows from zero to the value (where \[{{I}_{0}}\]is the steady state current)
A)
0.63\[{{I}_{0}}\]
done
clear
B)
0.50\[{{I}_{0}}\]
done
clear
C)
0.37 \[{{I}_{0}}\]
done
clear
D)
\[{{I}_{0}}\]
done
clear
View Answer play_arrow
question_answer 50) The horizontal component of .the earths magnetic field at a place is \[3\times {{10}^{-4}}T\] and the dip is \[{{\tan }^{-1}}\left( \frac{4}{3} \right).\] A metal rod of length 0.25 m placed in the north-south position and is moved at a constant speed of 10 cm/s towards the east. The emf induced in the rod will be
A)
zero
done
clear
B)
\[1\,\mu V\]
done
clear
C)
\[5\,\mu V\]
done
clear
D)
\[10\,\mu V\]
done
clear
View Answer play_arrow
question_answer 51) In \[\text{L-C-R}\] circuit, \[\text{R=100}\,\Omega \] When capacitance C is removed, the current lags behind the voltage by\[\frac{\pi }{3}.\] When inductance L is removed, the current leads the voltage by \[\frac{\pi }{3}.\]The impedance of the circuit is
A)
\[50\,\Omega \]
done
clear
B)
\[100\,\Omega \]
done
clear
C)
\[200\,\Omega \]
done
clear
D)
\[400\,\Omega \]
done
clear
View Answer play_arrow
question_answer 52) If 10000 V is applied across an X-ray tube, what will be the ratio of de-Broglie wavelength of the incident electrons to the shortest wavelength of X-ray produced? (e/m for electron is \[1.8\,\times {{10}^{11}}Ck{{g}^{-1}}\])
A)
1
done
clear
B)
0.1
done
clear
C)
0.2
done
clear
D)
0.3
done
clear
View Answer play_arrow
question_answer 53) An electron is accelerated through a potential difference of 1000 V. Its velocity is nearly
A)
\[3.8\,\times {{10}^{7}}m/s\]
done
clear
B)
\[\text{1}\text{.9 }\!\!\times\!\!\text{ 1}{{\text{0}}^{6}}\text{m/s}\]
done
clear
C)
\[\text{1}\text{.9 }\!\!\times\!\!\text{ 1}{{\text{0}}^{7}}\text{m/s}\]
done
clear
D)
\[\text{5}\text{.7 }\!\!\times\!\!\text{ 1}{{\text{0}}^{7}}\text{m/s}\]
done
clear
View Answer play_arrow
question_answer 54) A photon, an electron and uranium nucleus all have the same wavelength. The one with the most energy
A)
is the photon
done
clear
B)
is the electron
done
clear
C)
is the uranium nucleus
done
clear
D)
depends upon the wavelength and the properties of the particle
done
clear
View Answer play_arrow
question_answer 55) Which of the following statements is not correct?
A)
Photographic plates are sensitive to infrared rays
done
clear
B)
Photographic plates are sensitive to ultraviolet rays
done
clear
C)
Infra red rays are invisible but can cast shadows like visible light
done
clear
D)
Infrared photons have more energy than photons of visible light
done
clear
View Answer play_arrow
question_answer 56) Assuming photoemission to take place, the factor by which the maximum velocity of the emitted photoelectrons changes when the wavelength of the incident radiation is increased four times, is
A)
4
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
2
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 57) 57. A nucleus\[z{{X}^{A}}\] emits 9 \[\alpha -\]-particles and 5 \[\beta \]-particles. The ratio of total protons and neutrons in the final nucleus is
A)
\[\frac{Z-13}{(A-Z-23)}\]
done
clear
B)
\[\frac{Z-18}{(A-36)}\]
done
clear
C)
\[\frac{Z-13}{(A-36)}\]
done
clear
D)
\[\frac{Z-13}{(A-Z-13)}\]
done
clear
View Answer play_arrow
question_answer 58) If\[{{t}_{1/2}}\]is the half-life of a substance then\[{{t}_{3/4}}\]is the time in which substance
A)
decays\[\frac{\text{3}}{\text{4}}\text{th}\]
done
clear
B)
remains\[\frac{\text{3}}{\text{4}}\text{th}\]
done
clear
C)
decays\[\frac{1}{2}\text{th}\]
done
clear
D)
remains\[\frac{1}{2}\text{th}\]
done
clear
View Answer play_arrow
question_answer 59) An AC source of angular frequency \[\omega \] is fed across a resistor rand a capacitor C in series. The current registered is \[i.\] If now the frequency of source is changed to\[\omega /3\] (but maintaining the same voltage), the current in the circuit is found to be halved. Calculate the ratio of reactance to resistance at the original frequency\[\omega \].
A)
\[\sqrt{\frac{3}{5}}\]
done
clear
B)
\[\sqrt{\frac{2}{5}}\]
done
clear
C)
\[\sqrt{\frac{1}{5}}\]
done
clear
D)
\[\sqrt{\frac{4}{5}}\]
done
clear
View Answer play_arrow
question_answer 60) Susceptibility of Mg at 300 K is \[1.2\,\times {{10}^{-5}}\]. The temperature at which susceptibility will be \[1.8\,\times {{10}^{-5}}\] is
A)
450 K
done
clear
B)
200 K
done
clear
C)
375 K
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 61) In transistor, forward bias is always smaller than the reverse bias. The correct reason is
A)
to avoid excessive heating of transistor
done
clear
B)
to maintain a constant base current
done
clear
C)
to produce large voltage gain
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 62) In the Boolean algebra \[\overline{(\overline{A.}\overline{B}).}\] A equals to
A)
\[\overline{A+B}\]
done
clear
B)
A
done
clear
C)
A.B
done
clear
D)
A + B
done
clear
View Answer play_arrow
question_answer 63) The maximum peak to peak voltage of an AM wire is 24 mV and the minimum peak to peak voltage is 8 mV. The modulation factor is
A)
10%
done
clear
B)
20%
done
clear
C)
25%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 64) The focal length of objective lens and eyelens of a microscope are 4 cm and 8 cm respectively. If the least distance of distinct vision is 24 cm and object distance is 4.5 cm from the objective lens, then the magnifying power of the microscope will be
A)
18
done
clear
B)
32
done
clear
C)
64
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 65) When the length of a microscope tube increases, its magnifying power
A)
decreases
done
clear
B)
increases
done
clear
C)
does not change
done
clear
D)
may decrease or increase
done
clear
View Answer play_arrow
question_answer 66) On a rainy day, a small oil film on water show brilliant colours. This is due to
A)
dispersion of light
done
clear
B)
interference of light
done
clear
C)
absorption of light
done
clear
D)
scattering of light
done
clear
View Answer play_arrow
question_answer 67) Two waves having intensities in the ratio 25 : 4 produce interference. The ratio of the maximum to the minimum intensities is
A)
5 : 2
done
clear
B)
7 : 3
done
clear
C)
49 : 9
done
clear
D)
9 : 49
done
clear
View Answer play_arrow
question_answer 68) A Carnot engine whose low temperature reservoir is at \[7{}^\circ C\] has an efficiency of 50%. It is desired to increase the efficiency to 70%. By how many degrees should the temperature of the high temperature reservoir be increased?
A)
840 K
done
clear
B)
280 K
done
clear
C)
560 K
done
clear
D)
373 K
done
clear
View Answer play_arrow
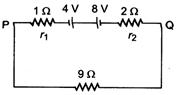
question_answer 69)
Two long straight wires are set parallel to each other. Each carries a current; in the same direction and the separation between them is 2r. The intensity of the magnetic field midway between them is
A)
\[\frac{{{\mu }_{0i}}}{r}\]
done
clear
B)
\[\frac{4{{\mu }_{0i}}}{r}\]
done
clear
C)
zero
done
clear
D)
\[\frac{{{\mu }_{0i}}}{4r}\]
done
clear
View Answer play_arrow
question_answer 70) In hydrogen atom, an electron is revolving in the orbit of radius \[0.53\,\overset{\text{o}}{\mathop{\text{A}}}\,\] with \[6.6\,\times {{10}^{15}}\] rotation/s. Magnetic field produced at the centre of the orbit is
A)
\[0.125\,Wb/{{m}^{2}}\]
done
clear
B)
\[1.25\,Wb/{{m}^{2}}\]
done
clear
C)
\[12.5\,Wb/{{m}^{2}}\]
done
clear
D)
\[125\,\,Wb/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 71) An electric bulb illuminates a plane surface. The intensity of illumination on the surface at the point 2m away from the bulb is \[5\times {{10}^{-4}}\] phot (lumen/\[c{{m}^{2}}\]). The line joining the bulb to the paint makes an angle of \[60{}^\circ \] with the normal to the surface. The intensity of the bulb in candela is
A)
\[40\sqrt{3}\]
done
clear
B)
40
done
clear
C)
20
done
clear
D)
\[40\times {{10}^{-7}}\]
done
clear
View Answer play_arrow
question_answer 72)
Two batteries of emfs 4 V and 8 V with internal resistances \[1\,\Omega \] and\[2\,\Omega \] are connected in a circuit with a resistance of \[9\,\Omega \]as shown in figure. The current and potential difference between the points P and Q are
A)
\[\frac{\text{1}}{\text{2}}\text{A}\,\text{and}\,\text{3}\,\text{V}\]
done
clear
B)
\[\frac{\text{1}}{6}\text{A}\,\text{and}\,4\,\text{V}\]
done
clear
C)
\[\frac{\text{1}}{9}\text{A}\,\text{and}\,9\,\text{V}\]
done
clear
D)
\[\frac{\text{1}}{2}\text{A}\,\text{and}\,12\,\text{V}\]
done
clear
View Answer play_arrow
question_answer 73) The energy required to accelerate a car from 10 m/s to 20 m/s is how many times the energy required to accelerate the car from rest to 10 m/s?
A)
Equal
done
clear
B)
4 times
done
clear
C)
2 times
done
clear
D)
3 times
done
clear
View Answer play_arrow
question_answer 74) A cell of internal resistance\[1.5\Omega \] and ofemfl.5V balances 500 cm on a potentiometer wire. If a wire of\[15\Omega \] is connected between the balance point and the cell, then the balance point will shift
A)
to zero
done
clear
B)
by 500 cm
done
clear
C)
by 750 cm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 75) The colour sequence in a carbon resistor is red, brown, orange and silver. The resistance of the resistor is
A)
\[21\times {{10}^{3}}\pm \,10%\]
done
clear
B)
\[23\times {{10}^{1}}\pm \,10%\]
done
clear
C)
\[21\times {{10}^{3}}\pm \,10%\]
done
clear
D)
\[12\times {{10}^{3}}\pm \,5%\]
done
clear
View Answer play_arrow
question_answer 76) A moving coil galvanometer has a resistance of \[50\Omega \] and gives full scale deflection for 10 mA. How could it be converted into an ammeter with a full scale deflection for 1 A?
A)
\[50/99\,\Omega \] in series
done
clear
B)
\[50/99\,\Omega \] in parallel
done
clear
C)
\[0.01\,\Omega \] in series
done
clear
D)
\[0.01\,\Omega \] in parallel
done
clear
View Answer play_arrow
question_answer 77) The moment of inertia of a body about a given axis is \[2.4\,\,kg-{{m}^{2}}\]. To produce a rotational kinetic energy of 750 J, an angular acceleration of \[5\,rad/{{s}^{2}}\] must be applied about that axis for
A)
6 s
done
clear
B)
5 s
done
clear
C)
4 s
done
clear
D)
3 s
done
clear
View Answer play_arrow
question_answer 78) A conducting sphere of radius R, and carrying a charge q is joined to a conducting sphere of radius 2R, and carrying a charge - 2q. The charge flowing between them will be
A)
\[-\frac{q}{3}\]
done
clear
B)
\[\frac{2q}{3}\]
done
clear
C)
\[q\]
done
clear
D)
\[\frac{4q}{3}\]
done
clear
View Answer play_arrow
question_answer 79) The potential at a point due to an electron dipole will be maximum and minimum when the angles between the axis of the dipole and the line joining the point to the dipole are respectively
A)
\[90{}^\circ \text{ }and\text{ }180{}^\circ \]
done
clear
B)
\[0{}^\circ \text{ }and\text{ }90{}^\circ \]
done
clear
C)
\[90{}^\circ \text{ }and\text{ }0{}^\circ \]
done
clear
D)
\[0{}^\circ \text{ }and\text{ }180{}^\circ \]
done
clear
View Answer play_arrow
question_answer 80) On all the six surfaces of a unit cube, equal tensile force of F is applied. The increase in length of each side will be (Y = Youngs modulus, \[\rho \]= Poissions ratio)
A)
\[\frac{F}{Y(1-\sigma )}\]
done
clear
B)
\[\frac{F}{Y(1+\sigma )}\]
done
clear
C)
\[\frac{F(1-2\sigma )}{Y}\]
done
clear
D)
\[\frac{F}{Y(1+2\sigma )}\]
done
clear
View Answer play_arrow
question_answer 81) If the tension on a wire is removed at once, then
A)
it will break
done
clear
B)
its temperature will reduce
done
clear
C)
there will be no change in its temperature
done
clear
D)
its temperature increases
done
clear
View Answer play_arrow
question_answer 82) The electric field due to an electric dipole at a distance r from its centre in axial position is E. If the dipole is rotated through an angle of \[90{}^\circ \] about its perpendicular axis, the electric field at the same point will be
A)
E
done
clear
B)
\[\frac{E}{4}\]
done
clear
C)
\[\frac{E}{2}\]
done
clear
D)
\[2E\]
done
clear
View Answer play_arrow
question_answer 83) Among the following properties describing diamagnetism, identify the property that is wrongly stated.
A)
Diamagnetic materials do not have permanent magnetic moment
done
clear
B)
Diamagnetism is explained in terms of electromagnetic induction
done
clear
C)
Diamagnetic materials have a small positive susceptibility
done
clear
D)
The magnetic moment of individual electrons neutralize each other
done
clear
View Answer play_arrow
question_answer 84) Stars are not visible in the day time because
A)
stars hide behind the sun
done
clear
B)
stars do not reflect sunrays during day
done
clear
C)
stars vanish during the day
done
clear
D)
atmosphere scatters sunlight into a blanket of extreme brightness through which faint stars cannot be visible
done
clear
View Answer play_arrow
question_answer 85) If pressure at half the depth of a lake is equal to 2/3 pressure at the bottom of the lake then what is the depth of the lake?
A)
10 m
done
clear
B)
20 m
done
clear
C)
60 m
done
clear
D)
30 m
done
clear
View Answer play_arrow
question_answer 86) The mass of the moon is\[\frac{1}{81}\] of the earth but its gravitational pull is\[\frac{1}{6}\]of the earth. It is due to the fact that
A)
the radius of the moon is \[\frac{81}{6}\]of the earth
done
clear
B)
the radius of the earth is \[\frac{9}{\sqrt{6}}\]of the moon
done
clear
C)
moon is the satellite of the earth
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 87) A moving coil galvanometer has 48 turns and area of coil is \[4\times {{10}^{-2}}{{m}^{2}}\]. If the magnetic field is 0.2 T, then to increase the current sensitivity by 25% without changing area (A) and field (B) the number of turns should become
A)
24
done
clear
B)
36
done
clear
C)
60
done
clear
D)
54
done
clear
View Answer play_arrow
question_answer 88) A magnet is parallel to a uniform magnetic field. If it is rotated by \[60{}^\circ \], the work done is 0.8 J. How much work is done in moving it \[30{}^\circ \] further?
A)
\[\text{0}\text{.8 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{7}}}\,\text{erg}\]
done
clear
B)
4.0 J
done
clear
C)
8 J
done
clear
D)
0.8 erg
done
clear
View Answer play_arrow
question_answer 89) An electric dipole is put in north-south direction in a sphere filled with water. Which of the following statements is correct?
A)
Electric flux is coming towards sphere
done
clear
B)
Electric flux is coming out of sphere
done
clear
C)
Electric flux entering into sphere and leaving the sphere are same
done
clear
D)
Water does not permit electric flux to enter into sphere
done
clear
View Answer play_arrow
question_answer 90) The plates of parallel plate capacitor are charged upto 100V. A 2 mm thick plate is inserted between the plates. Then to maintain the same potential difference, the distance between the plates is increased by 1.6 mm. The dielectric constant of the plate is
A)
5
done
clear
B)
1.25
done
clear
C)
4
done
clear
D)
2.5
done
clear
View Answer play_arrow
question_answer 91) The root mean square speed of hydrogen molecules of an ideal hydrogen gas kept in a gas chamber at \[0{}^\circ C\] is 3180 m/s. The pressure on the hydrogen gas is (density of hydrogen gas is \[8.99\,\times {{10}^{-2}}kg/{{m}^{3}},\] atmosphere \[=1.01\,\times {{10}^{5}}\,N/{{m}^{2}}\])
A)
1.0 atm
done
clear
B)
1.5 atm
done
clear
C)
2.0 atm
done
clear
D)
3.0 atm
done
clear
View Answer play_arrow
question_answer 92) Which of the following is the disadvantage of FM over AM?
A)
Larger band width requirement
done
clear
B)
Larger noise
done
clear
C)
Higher modulation power
done
clear
D)
Low efficiency
done
clear
View Answer play_arrow
question_answer 93)
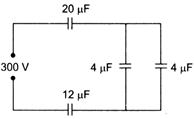
In the adjoining figure, four capacitors are shown with their respective capacities and the PD applied. The charge and the PD across the \[4\mu F\] capacitor will be
A)
\[600\,\mu C;\,150\,V\]
done
clear
B)
\[300\,\mu C;\,75\,V\]
done
clear
C)
\[800\,\mu C;\,200V\]
done
clear
D)
\[580\,\mu C;\,145V\]
done
clear
View Answer play_arrow
question_answer 94) What is the angle between the electric dipole moment and the electric field strength due to it on the equatorial line?
A)
\[0{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[180{}^\circ \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 95) An electron microscope is superior to an optical microscope in
A)
having better resolving power
done
clear
B)
being easy to handle
done
clear
C)
low cost
done
clear
D)
quickness of observation
done
clear
View Answer play_arrow
question_answer 96) An L-C-R series circuit with a resistance of 100 ft is connected to an AC source of 200 V and angular frequency 300 rad/s. When only the capacitor is removed, the current lags behind the voltage by \[60{}^\circ \]. When only the inductor is removed the current leads the voltage by \[60{}^\circ \] The average power dissipated is
A)
50 W
done
clear
B)
100 W
done
clear
C)
200 W
done
clear
D)
400 W
done
clear
View Answer play_arrow
question_answer 97) How much work must be done to pull apart the electron and the proton that make up the hydrogen atom, if the atom is initially in the state with n = 2?
A)
\[\text{13}\text{.6 }\!\!\times\!\!\text{ 1}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{J}\]
done
clear
B)
\[\text{3}\text{.6 }\!\!\times\!\!\text{ 1}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{J}\]
done
clear
C)
\[\text{1}\text{.51 }\!\!\times\!\!\text{ 1}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{J}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 98) If the current 3 A flowing in the primary coil ii made zero in 0.1 s. The emf induced in the secondary coil is 1.5 V. The mutual inductance between the coils is
A)
0.05 H
done
clear
B)
1.05 H
done
clear
C)
0.1 H
done
clear
D)
0.2 H
done
clear
View Answer play_arrow
question_answer 99) Radius of the curved road on national highway is R. Width of the road is b. The outer edge of the road is raised by h with respect to inner edge so that a car with velocity v can pass safe over it. The value of h is
A)
\[\frac{{{v}^{2}}b}{Rg}\]
done
clear
B)
\[\frac{v}{Rgb}\]
done
clear
C)
\[\frac{{{v}^{2}}R}{g}\]
done
clear
D)
\[\frac{{{v}^{2}}b}{R}\]
done
clear
View Answer play_arrow
question_answer 100) What is the (rms) value of an alternating current which when passed through a resistor produces heat which is thrice that of produced by a direct current of 2 A in the same resistor?
A)
6 A
done
clear
B)
2 A
done
clear
C)
3.46 A
done
clear
D)
0.66 A
done
clear
View Answer play_arrow
question_answer 101) The monomer units of starch are
A)
\[\alpha -\]glucose
done
clear
B)
\[\beta -\]glucose
done
clear
C)
pyranose
done
clear
D)
galactose
done
clear
View Answer play_arrow
question_answer 102) The only vitamin with metal atom in it, is
A)
vitamin\[-A\]
done
clear
B)
vitamin\[-K\]
done
clear
C)
vitamin\[-{{B}_{12}}\]
done
clear
D)
vitamin\[-E\]
done
clear
View Answer play_arrow
question_answer 103) Which of the following is used to make non-stick cookware?
A)
PVC
done
clear
B)
Polystyrene
done
clear
C)
Polyethylene terephthalate
done
clear
D)
Polytetrafluoro ethylene
done
clear
View Answer play_arrow
question_answer 104) Nylon-6 is made from
A)
1, 3-butadiene
done
clear
B)
chloroprene
done
clear
C)
adipic acid
done
clear
D)
carpolactum
done
clear
View Answer play_arrow
question_answer 105) The functional group which is found in amino acid is
A)
\[-COOH\]
done
clear
B)
\[-N{{H}_{2}}\]
done
clear
C)
\[-C{{H}_{3}}\]
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 106) The units of conductivity are
A)
\[oh{{m}^{-1}}\]
done
clear
B)
\[oh{{m}^{-1}}c{{m}^{-1}}\]
done
clear
C)
\[oh{{m}^{-2}}c{{m}^{2}}equi{{v}^{-1}}\]
done
clear
D)
\[oh{{m}^{-1}}c{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 107) The colloidal system in which the disperse phase and dispersion medium are both liquids is known as
A)
a gel
done
clear
B)
an aerosol
done
clear
C)
an emulsion
done
clear
D)
a foam
done
clear
View Answer play_arrow
question_answer 108) Which of the following is not an ore of magnesium?
A)
Gypsum
done
clear
B)
Dolomite
done
clear
C)
Magnesite
done
clear
D)
Carnallite
done
clear
View Answer play_arrow
question_answer 109) An example of water soluble vitamin is
A)
vitamin-D
done
clear
B)
vitamin-E
done
clear
C)
vitamin-A
done
clear
D)
vitamin-C
done
clear
View Answer play_arrow
question_answer 110) During smelting an additional substance is added which combines with impurities to form a fusible product which is known as
A)
mud
done
clear
B)
slag
done
clear
C)
flux
done
clear
D)
gangue
done
clear
View Answer play_arrow
question_answer 111) Isotonic solutions are the solutions having the same
A)
surface tension
done
clear
B)
vapour pressure
done
clear
C)
osmotic pressure
done
clear
D)
viscosity
done
clear
View Answer play_arrow
question_answer 112) Hydrogen bond is strongest in
A)
\[S-H\cdot \cdot \cdot \cdot \cdot O\]
done
clear
B)
\[O-H\cdot \cdot \cdot \cdot \cdot S\]
done
clear
C)
\[F-H\cdot \cdot \cdot \cdot \cdot F\]
done
clear
D)
\[O-H\cdot \cdot \cdot \cdot \cdot N\]
done
clear
View Answer play_arrow
question_answer 113)
Match List I and List II and pick out correct matching codes from the given choices. List I (Compound) List II (Structure) A \[Cl{{F}_{3}}\] 1. Square planar B \[PC{{l}_{5}}\] 2. Tetrahedral C \[I{{F}_{5}}\] 3. Trigonal by pramidal D \[CC{{l}_{4}}\] 4. Square pyradimal E \[Xe{{F}_{4}}\] 5. T-Shaped
A)
\[A-5,\,\,B-4,\,\,C-3,\,\,D-2,\,\,E-1\]
done
clear
B)
\[A-5,\,\,B-3,\,\,C-4,\,\,D-2,\,\,E-1\]
done
clear
C)
\[A-5,\,\,B-3,\,\,C-4,\,\,D-1,\,\,E-2\]
done
clear
D)
\[A-4,\,\,B-3,\,\,C-5,\,\,D-2,\,\,E-1\]
done
clear
View Answer play_arrow
question_answer 114) In which of the following pairs, the two species have identical bond order?
A)
\[N_{2}^{-},\,\,O_{2}^{2-}\]
done
clear
B)
\[N_{2}^{+},\,\,O_{2}^{-}\]
done
clear
C)
\[N_{2}^{-},\,\,O_{2}^{+}\]
done
clear
D)
\[O_{2}^{+},\,\,N_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 115) The mass of a photon with wavelength \[3.6\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\] is
A)
\[6.135\times {{10}^{-19}}\]
done
clear
B)
\[5.6135\times {{10}^{-29}}\]
done
clear
C)
\[6.100\times {{10}^{-19}}\]
done
clear
D)
\[6.135\times {{10}^{-29}}\]
done
clear
View Answer play_arrow
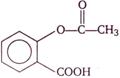
question_answer 116)
The following compound is used as
A)
an anti-inflammatory compound
done
clear
B)
analgesic
done
clear
C)
hypnotic
done
clear
D)
antiseptic
done
clear
View Answer play_arrow
question_answer 117) Presence of a nitro group in a benzene ring
A)
renders the ring basic
done
clear
B)
deactivates the ring towards nucleophilic substitution
done
clear
C)
deactivates the ring towards electrophilic substitution
done
clear
D)
activates the ring towards electrophilic substitution
done
clear
View Answer play_arrow
question_answer 118) Polymer used in bullet proof glass is
A)
PMMA
done
clear
B)
lexan
done
clear
C)
nomex
done
clear
D)
Kevlar
done
clear
View Answer play_arrow
question_answer 119) Which of the following is a pair of isobar?
A)
\[_{6}{{C}^{13}},\,{{\,}_{7}}{{N}^{13}}\]
done
clear
B)
\[_{6}{{C}^{13}},\,{{\,}_{7}}{{N}^{14}}\]
done
clear
C)
\[_{7}{{N}^{14}},\,{{\,}_{8}}{{O}^{16}}\]
done
clear
D)
\[_{7}{{N}^{13}},\,{{\,}_{8}}{{O}^{18}}\]
done
clear
View Answer play_arrow
question_answer 120) The nitrogen atom has 7 protons and 7 electrons. The nitride ion will have
A)
7 protons and 10 electrons
done
clear
B)
4 protons and 7 electrons
done
clear
C)
4 protons and 10 electrons
done
clear
D)
10 protons and 7 electrons
done
clear
View Answer play_arrow
question_answer 121) In which of the following the magnetic character is not correct?
A)
\[CuCl_{4}^{2}-1\]unpaired electron
done
clear
B)
\[[Fe{{({{H}_{2}}{{O}_{6}}]}^{2+}}-5\]unpaired electrons
done
clear
C)
\[{{[Zn{{(N{{H}_{3}})}_{2}}]}^{2+}}\]Diamagnetic
done
clear
D)
\[{{[Co{{F}_{6}}]}^{3-}}-4\]unpaired electrons
done
clear
View Answer play_arrow
question_answer 122) The complex used as an anticancer agent is
A)
\[mer-[CoC{{l}_{3}}{{(N{{H}_{3}})}_{3}}]\]
done
clear
B)
\[cis-[PtC{{l}_{2}}{{(N{{H}_{3}})}_{2}}]\]
done
clear
C)
\[cis-{{K}_{2}}[PtB{{r}_{2}}C{{l}_{2}}]\]
done
clear
D)
\[N{{a}_{2}}[Co{{(Cl)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 123) EDTA has coordination number
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 124) 3-pentanol is an example of
A)
primary alcohol
done
clear
B)
secondary alcohol
done
clear
C)
tertiary alcohol
done
clear
D)
aromatic alcohol
done
clear
View Answer play_arrow
question_answer 125) The incorrect IUPAC name is
A)
\[\underset{\text{2-methyl-3-butanone}}{\mathop{C{{H}_{3}}\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}}}\,\]
done
clear
B)
\[\underset{2,\,\,3-\text{dimethylpentane}}{\mathop{C{{H}_{3}}\underset{\begin{smallmatrix} || \\ C{{H}_{2}} \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ C{{H}_{2}}C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}}}\,\]
done
clear
C)
\[\underset{\text{2-pentyne}}{\mathop{C{{H}_{3}}-C\equiv C-C{{H}_{2}}-C{{H}_{3}}}}\,\]
done
clear
D)
\[\underset{\text{2-bromo-3-chloro-butane}}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}}}\,\]
done
clear
View Answer play_arrow
question_answer 126) Which alkene on ozonolysis gives\[C{{H}_{3}}C{{H}_{2}}CHO\] and\[C{{H}_{3}}COC{{H}_{3}}\]?
A)
\[C{{H}_{3}}C{{H}_{2}}CH=C{{(C{{H}_{3}})}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CH=CHC{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CH=CHC{{H}_{3}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CH=CHC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 127) Isopropyl bromide on Wurtz reaction gives
A)
hexane
done
clear
B)
propane
done
clear
C)
2, 3-dimethylbutane
done
clear
D)
neohexane
done
clear
View Answer play_arrow
question_answer 128) Which of the following represents the given mode of hybridization\[s{{p}^{2}}-s{{p}^{2}}-sp-sp\] from left to right?
A)
\[{{H}_{2}}C=CH-C\equiv N\]
done
clear
B)
\[HC\equiv C-C\equiv CH\]
done
clear
C)
\[{{H}_{2}}C=C=C{{H}_{2}}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 129) \[B{{F}_{3}}\]is
A)
electron-deficient compound
done
clear
B)
Lewis base
done
clear
C)
used as rocket fuel
done
clear
D)
ionic compound
done
clear
View Answer play_arrow
question_answer 130) \[C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ \underset{\centerdot \,\,\,\centerdot }{\mathop{O\,\,\mathbf{:}}}\, \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]and\[C{{H}_{2}}=\underset{\begin{smallmatrix} | \\ \underset{\centerdot \,\,\,\centerdot }{\mathop{\mathbf{:}O\mathbf{:}}}\, \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]are
A)
resonating structures
done
clear
B)
tautomers
done
clear
C)
geometrical isomers
done
clear
D)
optical isomers
done
clear
View Answer play_arrow
question_answer 131) Fluorobenzene can be synthesized in the laboratory
A)
from aniline by diazotization followed by heating the diazonium salt with\[HB{{F}_{4}}\]
done
clear
B)
by direct fluorination of benzene with \[{{F}_{2}}\] gas
done
clear
C)
by reacting bromobenzene with \[NaF\] solution
done
clear
D)
by heating phenol with \[HF\] and \[KF\]
done
clear
View Answer play_arrow
question_answer 132) Glucose reacts with X number of molecules of phenyl hydrazine to yield osazone. The value of\[X\]is
A)
three
done
clear
B)
two
done
clear
C)
one
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 133) The wavelength of which series lie towards the ultraviolet
A)
Lymann
done
clear
B)
Balmer
done
clear
C)
Paschen
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 134) A \[p-\]orbital accommodate maximum
A)
4 electrons
done
clear
B)
6 electrons
done
clear
C)
2 electrons with parallel spins
done
clear
D)
2 electrons with opposite spins
done
clear
View Answer play_arrow
question_answer 135) Ease of formation of the cation is favoured by
A)
lower value of ionization potential
done
clear
B)
lower value of electron affinity
done
clear
C)
higher value of electron affinity
done
clear
D)
lower value of electronegativity
done
clear
View Answer play_arrow
question_answer 136) What is the mole fraction of solute in \[2.5\,\,m\]aqueous solution?
A)
\[0.430\]
done
clear
B)
\[4.30\]
done
clear
C)
\[3.40\]
done
clear
D)
\[0.043\]
done
clear
View Answer play_arrow
question_answer 137) Which of the following carbon atoms is likely to possess tetrahedral geometry? \[{{H}_{2}}\overset{4}{\mathop{C}}\,=\overset{3}{\mathop{C}}\,H-\overset{2}{\mathop{C}}\,{{H}_{2}}-\overset{1}{\mathop{C}}\,OOH\]
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 138) The compound with an isopropyl group is
A)
2, 2, 3, 3-tetramethylpentane
done
clear
B)
2, 2-dimethylpentane
done
clear
C)
2, 2, 3, trimethylpentane
done
clear
D)
2-methylpentane
done
clear
View Answer play_arrow
question_answer 139) Borax is used as a cleansing agent because on dissolving in water, it gives
A)
alkaline solution
done
clear
B)
acidic solution
done
clear
C)
bleaching solution
done
clear
D)
amphoteric solution
done
clear
View Answer play_arrow
question_answer 140) Which is strongest Lewis acid?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[BB{{r}_{3}}\]
done
clear
D)
\[B{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 141) Which of the following has maximum unpaired \[d-\]electrons?
A)
\[Z{{n}^{2+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[N{{i}^{2+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 142) In the reaction \[C{{H}_{3}}C{{H}_{2}}COOH\xrightarrow{C{{l}_{2}},\,\,P}X\xrightarrow{alc.\,\,KOH}Y,\,\,Y\]is
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{2}}=CHCOOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CN\]
done
clear
D)
\[\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}C=COOH\]
done
clear
View Answer play_arrow
question_answer 143) Select the \[_{p}{{K}_{a}}\] value of the strongest acid.
A)
\[1.0\]
done
clear
B)
\[3.0\]
done
clear
C)
\[2.0\]
done
clear
D)
\[4.5\]
done
clear
View Answer play_arrow
question_answer 144) Hinsbergs reagent is
A)
benzene sulphonyl chloride
done
clear
B)
benzene sulphonic acid
done
clear
C)
phenyl isocyanide
done
clear
D)
benzene sulphonamide
done
clear
View Answer play_arrow
question_answer 145) Which of the following will not give a primary amine?
A)
\[C{{H}_{3}}CO{{H}_{2}}\xrightarrow{B{{r}_{2}},\,\,KOH}\]
done
clear
B)
\[C{{H}_{3}}CN\xrightarrow{LiAl{{H}_{4}}}\]
done
clear
C)
\[C{{H}_{3}}NHC\xrightarrow{LiAl{{H}_{4}}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\xrightarrow{LiAl{{H}_{4}}}\]
done
clear
View Answer play_arrow
question_answer 146) The product formed as a result of reaction of \[C{{H}_{3}}MgBr\] and\[C{{O}_{2}}\], on further hydrolysis gives
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[HCOOH\]
done
clear
C)
oxalic acid
done
clear
D)
acetic acid
done
clear
View Answer play_arrow
question_answer 147) The reaction \[RC{{H}_{2}}C{{H}_{2}}COOH\xrightarrow[B{{r}_{2}}]{\text{Red}\,\,P}RC{{H}_{2}}\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C}}\,HCOOH\]
A)
Reimer-Tiemann reaction
done
clear
B)
Hell Volhard Zelinsky reaction
done
clear
C)
Cannizaros reaction
done
clear
D)
Sandmeyers reaction
done
clear
View Answer play_arrow
question_answer 148) Which of the following compound is most reactive towards nucleophilic addition reaction?
A)
Ethanal
done
clear
B)
Propanal
done
clear
C)
Butanone
done
clear
D)
Propanone
done
clear
View Answer play_arrow
question_answer 149) For an equilibrium reaction, the rate constants for the forward and the backward reaction are \[2.38\times {{10}^{-4}}\] and \[8.15\times {{10}^{-5}}\] respectively. What will be the equilibrium constant for the reaction?
A)
\[92.2\]
done
clear
B)
\[29.2\]
done
clear
C)
\[20.2\]
done
clear
D)
\[2.92\]
done
clear
View Answer play_arrow
question_answer 150) Which acid is present in vinegar?
A)
Hydrochloric acid
done
clear
B)
Acetic acid
done
clear
C)
Citric acid
done
clear
D)
Tartaric acid
done
clear
View Answer play_arrow
question_answer 151) Which one of the following types of drugs reduces fever?
A)
Analgesics
done
clear
B)
Antipyretics
done
clear
C)
Aspirin
done
clear
D)
Tranquillizers
done
clear
View Answer play_arrow
question_answer 152) In\[HCO{{O}^{-}}\], the two carbon-oxygen bonds are found to be of equal length. What is the reason for this?
A)
The anion is obtained by the removal of a proton from the acid molecule
done
clear
B)
Electronic orbitals of carbon atoms are hybridized
done
clear
C)
The \[C=O\] bond is weaker than \[C-O\] bond
done
clear
D)
The anion \[HCO{{O}^{-}}\] has two resonating Structures
done
clear
View Answer play_arrow
question_answer 153) In a set of reactions, acetic acid yielded product \[D.C{{H}_{3}}COOH\xrightarrow{SOC{{l}_{2}}}A\xrightarrow[Anhy.\,\,AlC{{l}_{3}}]{Benzene}\]\[B\xrightarrow{HCN}C\xrightarrow{HOH}D\]. The structure of\[D\]would be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 154)
How many bonds are there in
A)
\[14\sigma ,\,\,8\pi \]
done
clear
B)
\[18\sigma ,\,\,8\pi \]
done
clear
C)
\[19\sigma ,\,\,4\pi \]
done
clear
D)
\[14\sigma ,\,\,2\pi \]
done
clear
View Answer play_arrow
question_answer 155) Principal, azimuthal and magnetic quantum numbers are respectively related to
A)
size, orientation and shape
done
clear
B)
size, shape and orientation
done
clear
C)
shape, size and orientation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 156) Paulis exclusion principle states that
A)
the nucleus of an atom is negatively charged
done
clear
B)
electrons revolve around the nucleus in circular orbits
done
clear
C)
electrons enter into lowest energy orbitals
done
clear
D)
no two electrons in an atom can have all the four quantum numbers identical
done
clear
View Answer play_arrow
question_answer 157) The geometry of \[XeO{{F}_{2}}\] is
A)
pyramidal
done
clear
B)
T-shaped
done
clear
C)
octahedral
done
clear
D)
tetrahedral
done
clear
View Answer play_arrow
question_answer 158) The noble gas compound prepared by Harriett was
A)
\[Xe{{O}_{3}}\]
done
clear
B)
\[XePt{{F}_{6}}\]
done
clear
C)
\[Kr{{F}_{2}}\]
done
clear
D)
\[Xe{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 159) Which of the following has highest ionization enthalpy?
A)
\[P\]
done
clear
B)
\[N\]
done
clear
C)
\[As\]
done
clear
D)
\[Sb\]
done
clear
View Answer play_arrow
question_answer 160) \[X\] is heated with soda lime and gives ethane. \[X\] is
A)
ethanoic acid
done
clear
B)
methanoic acid
done
clear
C)
propanoic acid
done
clear
D)
Either (a) or (c)
done
clear
View Answer play_arrow
question_answer 161) The correct order regarding the electronegativity of hybrid orbitals of carbon is
A)
\[sp<s{{p}^{2}}>s{{p}^{3}}\]
done
clear
B)
\[sp<s{{p}^{2}}<s{{p}^{3}}\]
done
clear
C)
\[sp>s{{p}^{2}}<s{{p}^{3}}\]
done
clear
D)
\[sp>s{{p}^{2}}>s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 162) Which of the following gas mixture is used by the divers inside the sea?
A)
\[{{O}_{2}}+He\]
done
clear
B)
\[{{O}_{2}}+Xe\]
done
clear
C)
\[{{O}_{2}}+Ar\]
done
clear
D)
\[{{O}_{2}}+{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 163) The laughing gas is
A)
nitrogen oxide
done
clear
B)
nitric oxide
done
clear
C)
nitrogen trioxide
done
clear
D)
nitrogen pentoxide
done
clear
View Answer play_arrow
question_answer 164) The number of nodal plane \[5\,\,d\] orbital has is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 165) The atomic number of \[Ni\] and \[Cu\] are \[28\] and \[29\] respectively. The electronic configuration\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] represents
A)
\[Ni\]
done
clear
B)
\[N{{i}^{2+}}\]
done
clear
C)
\[C{{u}^{2+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 166) How many ions are produced from\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]in solution?
A)
\[6\]
done
clear
B)
\[4\]
done
clear
C)
\[3\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 167) Among the alkali metals, cesium is the most reactive because
A)
its incomplete shell is nearest to the nucleus
done
clear
B)
its has a single electron in the valence shell
done
clear
C)
it is the heaviest alkali metal
done
clear
D)
the outermost electron is more loosely bound than the outermost electron of the other alkali metals
done
clear
View Answer play_arrow
question_answer 168) The ionic mobility of alkali metal ions in aqueous solution is maximum for
A)
\[{{K}^{+}}\]
done
clear
B)
\[R{{b}^{+}}\]
done
clear
C)
\[L{{i}^{+}}\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 169) Gypsum on heating to \[390\,\,K\] gives
A)
\[CaS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}\]
done
clear
C)
\[CaS{{O}_{4}}\cdot \frac{1}{2}{{H}_{2}}O\]
done
clear
D)
\[S{{O}_{3}}\]and\[CaO\]
done
clear
View Answer play_arrow
question_answer 170) Epsom salt is
A)
\[MgS{{O}_{4}}\cdot 7{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}\cdot {{H}_{2}}O\]
done
clear
C)
\[MgS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
D)
\[BaS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 171) The temperature at which \[28\,\,g\] of \[{{N}_{2}}\] will occupy a volume of \[10.0\,\,L\] at\[2.46\,\,atm\]
A)
\[299.6\,\,K\]
done
clear
B)
\[{{0}^{o}}C\]
done
clear
C)
\[273\,\,K\]
done
clear
D)
\[{{10}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 172) Para and ortho hydrogen differ in
A)
atomic number
done
clear
B)
atomic mass
done
clear
C)
spins of protons
done
clear
D)
number of neutrons
done
clear
View Answer play_arrow
question_answer 173) Atomic orbitals of carbon in carbon dioxide are
A)
\[sp-\]hybridized
done
clear
B)
\[s{{p}^{3}}d-\]hybridized
done
clear
C)
\[s{{p}^{2}}-\]hybridized
done
clear
D)
\[s{{p}^{3}}-\]hybridized
done
clear
View Answer play_arrow
question_answer 174) The appearance of colour in solid alkali metal halides is generally due to
A)
Schottky defect
done
clear
B)
Frenkel defect
done
clear
C)
Interstitial position
done
clear
D)
\[F-\]centres
done
clear
View Answer play_arrow
question_answer 175) Which among the following factors is most important in making fluorine the strongest oxidizing agent?
A)
Electron gain enthalpy
done
clear
B)
Ionization enthalpy
done
clear
C)
Hydration enthalpy
done
clear
D)
Bond dissociation enthalpy
done
clear
View Answer play_arrow
question_answer 176) How many coulombs are required to deposit \[50\,\,g\] of aluminium when the electrode reaction is \[A{{l}^{3+}}+3{{e}^{-}}\xrightarrow{{}}Al\]
A)
\[536111\,\,C\]
done
clear
B)
\[536.111\,\,C\]
done
clear
C)
\[96500\,\,C\]
done
clear
D)
\[38600\,\,C\]
done
clear
View Answer play_arrow
question_answer 177) In\[Cu\,\,(At.\,\,No.29)\]
A)
13 electrons have spin in one direction and 16 electrons in other direction
done
clear
B)
14 electrons have spin one direction and 15 electrons in other direction
done
clear
C)
one electron can have spin only in the clockwise direction
done
clear
D)
None of the above is correct
done
clear
View Answer play_arrow
question_answer 178) The boiling point of water is\[{{100}^{o}}C\]. What will be the boiling point of an aqueous solution containing \[0.6\,\,g\] of urea (molar mass\[=60\]) in \[100\,\,g\] of water?(\[{{K}_{b}}\]for water\[=0.52\,\,K/m\])
A)
\[{{100.052}^{o}}C\]
done
clear
B)
\[{{101.052}^{o}}C\]
done
clear
C)
\[{{120.52}^{o}}C\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 179) Sodium metal crystallizes in body centred cubic lattice with cell edge\[4.29\overset{\text{o}}{\mathop{\text{A}}}\,\] . What is the radius of sodium atom?
A)
\[2.86\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[6.81\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1.68\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1.86\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 180) Hydrogen sulphide is acidic while water is neutral. The reason is
A)
molecular weight of \[{{H}_{2}}S\] is more than \[{{H}_{2}}O\]
done
clear
B)
water molecules associate, while \[{{H}_{2}}S\] molecules do not
done
clear
C)
\[H-S\]bond is weaker than \[H-O\] bond due to the bigger size of \[S-\]atom
done
clear
D)
S-atoms has less affinity for hydrogen atom than \[O\] atom has for it
done
clear
View Answer play_arrow
question_answer 181) Percentage of cation in ammonium dichromate \[{{(N{{H}_{4}})}_{2}}C{{r}_{2}}{{O}_{7}}\] is
A)
\[41.29%\]
done
clear
B)
\[14.29%\]
done
clear
C)
\[20.29%\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 182) Identify the compounds \[A\] and \[B\] in the following reaction sequence. \[Ca{{C}_{2}}(s)+{{H}_{2}}O(l)\xrightarrow{{}}A(g)\xrightarrow[HgS{{O}_{4}}]{{{H}_{2}}S{{O}_{4}}}B(l)\]
A)
\[A\]is ethylene, \[B\] is acetaldehyde
done
clear
B)
\[A\]is acetylene, \[B\] is propionaldehyde
done
clear
C)
\[A\]is ethane, \[B\] is ethanol
done
clear
D)
\[A\]is acetylene, \[B\] is acetaldehyde
done
clear
View Answer play_arrow
question_answer 183) The compound formed in the positive test for nitrogen with the Lassaigne solution of an organic compound is
A)
\[F{{e}_{4}}{{[Fe(C{{N}_{6}})]}_{3}}\]
done
clear
B)
\[N{{a}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[Fe{{(CN)}_{3}}\]
done
clear
D)
\[N{{a}_{4}}[Fe{{(CN)}_{5}}COS]\]
done
clear
View Answer play_arrow
question_answer 184) The arrangement of\[{{(C{{H}_{3}})}_{3}}C-,\,\,{{(C{{H}_{3}})}_{2}}CH-,\]\[C{{H}_{3}}C{{H}_{2}}-\]when attached to benzene or unsaturated group in increasing order of inductive effect is
A)
\[{{(C{{H}_{3}})}_{3}}C-<{{(C{{H}_{3}})}_{3}}CH-<C{{H}_{3}}C{{H}_{2}}-\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}-<{{(C{{H}_{3}})}_{2}}CH-<{{(C{{H}_{3}})}_{3}}C-\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CH-<{{(C{{H}_{3}})}_{3}}C-<C{{H}_{3}}C{{H}_{2}}-\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}C-<C{{H}_{3}}C{{H}_{2}}-<{{(C{{H}_{3}})}_{2}}CH-\]
done
clear
View Answer play_arrow
question_answer 185) Pieces of wood burn faster than a log of wood of the same mass because
A)
surface area of log of wood is larger and needs more time to burn
done
clear
B)
pieces of wood have large surface area
done
clear
C)
all pieces of wood catch fire at the same time
done
clear
D)
block of wood has higher density than pieces of the same wood
done
clear
View Answer play_arrow
question_answer 186) Bravais lattices are of
A)
8 types
done
clear
B)
9 types
done
clear
C)
12 types
done
clear
D)
14 types
done
clear
View Answer play_arrow
question_answer 187) In the following nuclear reaction, the other product is \[_{52}T{{e}^{130}}{{+}_{1}}{{H}^{2}}{{\xrightarrow{{}}}_{53}}{{I}^{131}}+?\]
A)
positron
done
clear
B)
one neutron
done
clear
C)
proton
done
clear
D)
alpha particle
done
clear
View Answer play_arrow
question_answer 188) The number of a and p-particles emitted in the nuclear reaction \[_{90}T{{h}^{228}}{{\xrightarrow{{}}}_{83}}B{{i}^{212}}\]are
A)
\[4\alpha \]and\[1\beta \]
done
clear
B)
\[3\alpha \]and\[7\beta \]
done
clear
C)
\[8\alpha \]and\[1\beta \]
done
clear
D)
\[4\alpha \]and\[7\beta \]
done
clear
View Answer play_arrow
question_answer 189) Schottky defect in crystals is observed when
A)
density of crystal is increased
done
clear
B)
an ion leaves its normal site and occupies an interstitial site
done
clear
C)
equal number of cations and anions are missing from the lattice
done
clear
D)
unequal number of cations and anions are missing from the lattice
done
clear
View Answer play_arrow
question_answer 190) What happens when an egg is kept in saturated solution of \[NaCl\] after removing its hard shell in\[dil\,\,HCl\]?
A)
Egg will swell
done
clear
B)
Egg will shrink
done
clear
C)
Egg will remain same
done
clear
D)
Egg will first shrink and then swell
done
clear
View Answer play_arrow
question_answer 191) Which of the following is the expression of Raoults law? (\[p=\]vapour pressure of pure solvent, \[{{p}_{s}}=\]vapour pressure of the solution)
A)
\[\frac{p-{{p}_{s}}}{p}=\frac{n}{n+N}\]
done
clear
B)
\[\frac{{{p}_{s}}-p}{p}=\frac{N}{N+n}\]
done
clear
C)
\[\frac{p-{{p}_{s}}}{{{p}_{s}}}=\frac{N}{N-n}\]
done
clear
D)
\[\frac{{{p}_{s}}-p}{{{p}_{s}}}=\frac{N-n}{N}\]
done
clear
View Answer play_arrow
question_answer 192) A catalyst alter the rate of reaction by
A)
altering enthalpy
done
clear
B)
altering internal energy
done
clear
C)
altering energy of activation
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 193) The noble gas which can diffuse through rubber and glass easily is
A)
\[Xe\]
done
clear
B)
\[Ne\]
done
clear
C)
\[Ar\]
done
clear
D)
\[He\]
done
clear
View Answer play_arrow
question_answer 194)
Among the following molecule (i) \[Xe{{O}_{3}}\] (ii)\[Xe{{O}_{4}}\] (iii)\[Xe{{F}_{6}}\]
those having same number of lone pairs on\[Xe\] are
A)
(i) and (iii) only
done
clear
B)
(i) and (ii) only
done
clear
C)
(ii) and (iii) only
done
clear
D)
(i), (ii) and (iii)
done
clear
View Answer play_arrow
question_answer 195)
In the following halogen compounds, which compound undergoes fastest \[{{S}_{N}}1\] reaction?
A)
(i)
done
clear
B)
(ii)
done
clear
C)
(iii)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 196) \[UV\]radiations brings about
A)
skin cancer
done
clear
B)
mouth cancer
done
clear
C)
lung cancer
done
clear
D)
liver cancer
done
clear
View Answer play_arrow
question_answer 197) Sublimation is a process in which a solid
A)
changes into vapour form
done
clear
B)
changes into another allotropic form
done
clear
C)
changes into liquid form
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 198) Pollution is
A)
removal of top soil
done
clear
B)
release of toxic/undesirable materials in environment
done
clear
C)
conservation of energy
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 199) Gas released during Bhopal tragedy was
A)
methyl isocyanate
done
clear
B)
potassium isothiocyanate
done
clear
C)
sodium isothiocyanate
done
clear
D)
ethyl isothiocyanate
done
clear
View Answer play_arrow
question_answer 200) In\[AgBr\], these can occur
A)
Only Schottky defect
done
clear
B)
Only Frenkel defect
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 201) Plasmodesmata connections help in
A)
cytoplasmic streaming
done
clear
B)
synchronous mitotic divisions
done
clear
C)
locomotion of unicellular organisms
done
clear
D)
movement of substances between cells
done
clear
View Answer play_arrow
question_answer 202) The quiescent centre in root meristem serves as
A)
site for storage of food, which is utilized during maturation
done
clear
B)
reservoir of growth hormones
done
clear
C)
reserve for replenishment of damaged cells of the meristem
done
clear
D)
region for absorption of water
done
clear
View Answer play_arrow
question_answer 203) Azolla is used as a biofertilizer because it
A)
multiplies very fast to produce massive biomass
done
clear
B)
has association of nitrogen-fixing Rhizobium
done
clear
C)
has association of nitrogen-fixing cyanobacteria
done
clear
D)
has association of mycorrhiza
done
clear
View Answer play_arrow
question_answer 204) A person is wearing spectacles with concave lenses for correcting vision. While not using the glasses, the image of a distant object in his case will be formed
A)
on the blind spot
done
clear
B)
behind the retina
done
clear
C)
in front of the retina
done
clear
D)
on the yellow spot
done
clear
View Answer play_arrow
question_answer 205) Which one of the following codon codes for the same information as UGC?
A)
UGU
done
clear
B)
UGA
done
clear
C)
UAG
done
clear
D)
UGG
done
clear
View Answer play_arrow
question_answer 206) People recovering from long illness are often advised to include the alga Spirulina in their diet because it
A)
makes the food easy to digest
done
clear
B)
is rich in proteins
done
clear
C)
has antibiotic properties
done
clear
D)
restores the intestinal microflora
done
clear
View Answer play_arrow
question_answer 207) The phase of menstrual cycle in humans that lasts for 7-8 days is
A)
follicular phase
done
clear
B)
ovulatory phase
done
clear
C)
luteal phase
done
clear
D)
menstruation
done
clear
View Answer play_arrow
question_answer 208) Restriction enzymes
A)
are endonucleases, which cleave DNA at specific sites
done
clear
B)
make DNA complementary to an existing DNA or RNA
done
clear
C)
cut or join DNA fragments
done
clear
D)
are required in vector less direct gene transfer
done
clear
View Answer play_arrow
question_answer 209) Excessive stimulation of vagus nerve in humans may lead to
A)
hoarse voice
done
clear
B)
peptic ulcers
done
clear
C)
efficient digestion of proteins
done
clear
D)
irregular contraction of diaphragm
done
clear
View Answer play_arrow
question_answer 210) An example of competitive inhibition of an enzyme is the inhibition of
A)
succinic dehydrogenase by malonic acid
done
clear
B)
cytochrome oxidase by cyanide
done
clear
C)
hexokinase by glucose-6-phosphate
done
clear
D)
carbonic anhydrase by carbon dioxide
done
clear
View Answer play_arrow
question_answer 211) A person passes much urine and drinks much water but his blood glucose level is normal. This conditions may be the result of
A)
a reduction in insulin secretion from pancreas
done
clear
B)
a reduction in vasopressin secretion from posterior pituitary
done
clear
C)
a fall in the glucose concentration in urine
done
clear
D)
an increase in secretion of glucagon
done
clear
View Answer play_arrow
question_answer 212) Biological Oxygen Demand (BOD) is a measure of
A)
industrial wastes poured into water bodies
done
clear
B)
extent to which water is polluted with organic compound
done
clear
C)
amount of carbon monoxide inseparably combined with haemoglobin
done
clear
D)
amount of oxygen needed by green plants during night
done
clear
View Answer play_arrow
question_answer 213) Somaclonal variation is seen in
A)
tissue culture grown plants
done
clear
B)
apomicts
done
clear
C)
polyploids
done
clear
D)
vegetatiely propagated plants
done
clear
View Answer play_arrow
question_answer 214) Among rust, smut and mushroom, all the three
A)
are pathogens
done
clear
B)
are saprobes
done
clear
C)
bear ascocarps
done
clear
D)
bear basidiocarps
done
clear
View Answer play_arrow
question_answer 215) In prokaryotes, chromatophores are
A)
specialized granules responsible for coloration of cells
done
clear
B)
structures responsible for organizing the shape of the organism
done
clear
C)
incusion bodies lying free inside the cells for carrying out various metabolic activities
done
clear
D)
internal membrane systems that may become extensive and complex in photosynthetic bacteria
done
clear
View Answer play_arrow
question_answer 216) A scion is grafted to a stock. The quality of fruits produced will be determined by the genotype of
A)
stock
done
clear
B)
scion
done
clear
C)
Both (a) and (b)
done
clear
D)
Neither (a) nor (b)
done
clear
View Answer play_arrow
question_answer 217) The Montreal Protocol refers to
A)
persistent organic pollutants
done
clear
B)
global warming and climate change
done
clear
C)
substances that deplete the ozone layer
done
clear
D)
biosafety of genetically modified organisms
done
clear
View Answer play_arrow
question_answer 218) Keystone species deserve protection because these
A)
are capable of surviving in harsh environmental conditions
done
clear
B)
Indicate presence of certain minerals in the soil
done
clear
C)
have become rare due to over exploitation
done
clear
D)
play an important role in supporting other species
done
clear
View Answer play_arrow
question_answer 219) Grafting is successful in dicots but not in monocots because the dicots have
A)
vascular bundles arranged in a ring
done
clear
B)
cambium for secondary growth
done
clear
C)
vessels with elements arranged end to end
done
clear
D)
cork cambium
done
clear
View Answer play_arrow
question_answer 220) In the sieve elements, which one of the following is the most likely function of P-proteins?
A)
Deposition of callose on sieve plates
done
clear
B)
Providing energy for active translocation
done
clear
C)
Autolyric enzymes
done
clear
D)
Sealing off mechanism on wounding
done
clear
View Answer play_arrow
question_answer 221) A normal woman whose father was colurblind, is married to a normal man. The sons would be
A)
75% colourblind
done
clear
B)
50% colourblind
done
clear
C)
all normal
done
clear
D)
all colourblind
done
clear
View Answer play_arrow
question_answer 222) Which one of the following precedes re-formation of the nuclear envelope during M phase of the cell cycle?
A)
Decondensation from chromosomes and reassembly of the nuclear lamina
done
clear
B)
Transcription from chromosome and reassembly of the nuclear lamina
done
clear
C)
Formation of the contractile ring and formation of the phragmoplast
done
clear
D)
Formation of the contractile ring and transcription from chromosomes
done
clear
View Answer play_arrow
question_answer 223) The type of epithelial cells, which line the inner surface of Fallopian tubes, bronchioles and small bronchi, are known as
A)
squamous epithelium
done
clear
B)
columnar epithelium
done
clear
C)
ciliated epithelium
done
clear
D)
cubical epithelium
done
clear
View Answer play_arrow
question_answer 224) Which one of the following pairs is correctly matched?
A)
Rhizobium -Parasite in the roots of leguminous plants
done
clear
B)
Mycorrhizae - Mineral uptake from soil
done
clear
C)
Yeast - Production of biogas
done
clear
D)
Myxomycetes - The disease ringworm
done
clear
View Answer play_arrow
question_answer 225) A cricket player is fast chasing a ball in the field. Which one of the following groups of bones are directly contributing in this movement?
A)
Femur, malleus, tibia, metatarsals
done
clear
B)
Pelvis, ulna, patella, tarsals
done
clear
C)
Sternum, femur, tibia, fibula
done
clear
D)
Tarsals, femur, metatarsals, tibia
done
clear
View Answer play_arrow
question_answer 226) Viruses that infect bacteria multiply and cause their lysis, are called
A)
lysozymes
done
clear
B)
lipolytic
done
clear
C)
lyric
done
clear
D)
lysogenic
done
clear
View Answer play_arrow
question_answer 227) Pollution from animal excreta and organic waste from kitchen can be most profitably minimized by
A)
storing them in underground storage tanks
done
clear
B)
using them for producing biogas
done
clear
C)
vermiculture
done
clear
D)
using them directly as bio-fertilizers
done
clear
View Answer play_arrow
question_answer 228) Mating of an organism to a double recessive in order to determine whether it is homozygous or heterozygous for a character under consideration is called
A)
reciprocal cross
done
clear
B)
test cross
done
clear
C)
dihybrid cross
done
clear
D)
back cross
done
clear
View Answer play_arrow
question_answer 229) Which one of the following pairs of features is a good example of polygenic inheritance?
A)
Human height and skin colour
done
clear
B)
ABO blood group in humans and flower colour of Mirabilis jalapa
done
clear
C)
Hair pigment of mouse and tongue rolling in humans
done
clear
D)
Human eye colour and sickle cell anaemia
done
clear
View Answer play_arrow
question_answer 230) A lizard-like member of Reptilia is string on a tree with its tail coiled around a twig. This animal could be
A)
Hemidactylus showing sexual dimorphism
done
clear
B)
Varanus showing mimicry
done
clear
C)
Calotes (garden lizard) showing camouflage
done
clear
D)
Chamaeleon showing protective colouration
done
clear
View Answer play_arrow
question_answer 231) All mammals without any exception are characterised by
A)
vivipariry and biconcave red blood cells extra
done
clear
B)
abdominal tests and a four-chambered heart
done
clear
C)
heterodont teeth and 12 pairs of cranial nerves
done
clear
D)
a muscular diaphragm and milk producing glands
done
clear
View Answer play_arrow
question_answer 232) During the transmission of nerve impulse through a nerve fibre, the potential on the inner side of the plasma membrane has which type of electric charge?
A)
First negative, then positive and again back to negative
done
clear
B)
First positive, then negative and continue to be negative
done
clear
C)
First negative, then positive and continue to be positive
done
clear
D)
First positive, then negative and again back to positive
done
clear
View Answer play_arrow
question_answer 233) If you are asked to classify the various algae into distinct groups, which of the following characters you should choose?
A)
Types of pigments present in the cell
done
clear
B)
Nature of stored food materials in the cell
done
clear
C)
Structure organization of thallus
done
clear
D)
Chemical composition of the cell wall
done
clear
View Answer play_arrow
question_answer 234) A plant require magnesium (Mg) for
A)
holding cells together
done
clear
B)
protein synthesis
done
clear
C)
chlorophyll synthesis
done
clear
D)
cell wall development
done
clear
View Answer play_arrow
question_answer 235) Ultrasound of how much frequency is beamed into human body for sonography?
A)
30 to 45 MHz
done
clear
B)
15 to 30 MHz
done
clear
C)
1 to 5 MHz
done
clear
D)
45 to 70 MHz
done
clear
View Answer play_arrow
question_answer 236) Which one of the following pairs of structures distinguished a nerve cell from other types of cell?
A)
Perikaryon and dendrites
done
clear
B)
Vacuoles and fibres
done
clear
C)
Flagellum and medullary sheath
done
clear
D)
Nucleus and mitochondria
done
clear
View Answer play_arrow
question_answer 237) All enzymes of TCA cycle are located in the mitochondrial matrix except one, which is located in inner mitochondrial membranes in eukaryotes and in cytosol in prokaryotes. This enzyme is
A)
lactate dehydrogenase
done
clear
B)
isocitrate dehydrogenase
done
clear
C)
malate dehydrogenase
done
clear
D)
succinate dehydrogenase
done
clear
View Answer play_arrow
question_answer 238) When two species of different genealogy come to resemble each other as a result of adaptation the phenomenon is termed as
A)
divergent evolution
done
clear
B)
microevolution
done
clear
C)
co-evolution
done
clear
D)
convergent evolution
done
clear
View Answer play_arrow
question_answer 239) Which one of the following is a matching pair of a body feature and the animal possessing it?
A)
Post-anal tail - Octopus
done
clear
B)
Ventral central nervous - Leech system
done
clear
C)
Pharyngeal gills slits absent - Chamaeleon m embryo
done
clear
D)
Ventral heart - Scorpion
done
clear
View Answer play_arrow
question_answer 240) Bowmans glands are located in the
A)
proximal end of uriniferous tubules
done
clear
B)
anterior pituitary
done
clear
C)
female reproductive system of cockroach
done
clear
D)
olfactory epithelium of our nose
done
clear
View Answer play_arrow
question_answer 241) Which one of the following pairs, is not correctly matched?
A)
Abscisic acid - Stomatal closure
done
clear
B)
Gibberellic acid - Leaf fall
done
clear
C)
Cytokinin - Cell division
done
clear
D)
IAA - Cell wall elongation
done
clear
View Answer play_arrow
question_answer 242) Which one of the following element is not an essential micronutrient for plant growth?
A)
Manganese
done
clear
B)
Zinc
done
clear
C)
Copper
done
clear
D)
Calcium
done
clear
View Answer play_arrow
question_answer 243) Two cells A and B are contiguous. Cell A has osmotic pressure 10 at m, turgor pressure-7 at m and diffusion pressure deficit 3 at m. Cell B has osmotic pressure 8 at m, turgor pressure 3 at m and diffusion pressure deficit 5 atm. The result will be
A)
movement of water from cell B to A
done
clear
B)
no movement of water
done
clear
C)
equilibrium between the two
done
clear
D)
movement of water from cell A to B
done
clear
View Answer play_arrow
question_answer 244) Which one of the following is surrounded by a callose wall?
A)
Microspore mother cell
done
clear
B)
Male gemete
done
clear
C)
Egg
done
clear
D)
Pollen grain
done
clear
View Answer play_arrow
question_answer 245) Male gametes in angiosperms are formed by the division of
A)
microspore
done
clear
B)
generative cell
done
clear
C)
vegetative cell
done
clear
D)
microspore mother cell
done
clear
View Answer play_arrow
question_answer 246) Which one of the following is not a bioindicator of water pollution?
A)
Sludge-worm
done
clear
B)
Blood-worm
done
clear
C)
Stone fly
done
clear
D)
Sewage fungus
done
clear
View Answer play_arrow
question_answer 247) Differentiation of organs and tissues in a developing organism, is associated with
A)
development mutations
done
clear
B)
differential expression of genes
done
clear
C)
lethal mutations
done
clear
D)
deletion of genes
done
clear
View Answer play_arrow
question_answer 248) Industrial melanism as observed in peppered moth proves that
A)
the true black melanic form arise by a recurring random mutation
done
clear
B)
the melanic form of the moth has no selective advantage over lighter form in industrial area
done
clear
C)
the lighter-form moth has no selective advantage either in polluted industrial area or non-polluted area
done
clear
D)
melanism is a pollution-generated feature
done
clear
View Answer play_arrow
question_answer 249) A genetically engineered microorganism used successfully in bioremediation of oil spills is a species of
A)
Pseudomonas
done
clear
B)
Trichoderma
done
clear
C)
Xanthomonas
done
clear
D)
Bacillus
done
clear
View Answer play_arrow
question_answer 250) About 96 per cent of the mass of every living organism is composed of just six elements including carbon, hydrogen, nitrogen, oxygen,
A)
phosphorus and sulphur
done
clear
B)
sulphur and magnesium
done
clear
C)
magnesium and sodium
done
clear
D)
calcium and phosphorus
done
clear
View Answer play_arrow
question_answer 251) Which one of the following pairs is mismatched?
A)
Pila globosa - Pearl
done
clear
B)
Apis indica - Honey
done
clear
C)
Kenia lacca - Lac
done
clear
D)
Bombyx mori - Silk
done
clear
View Answer play_arrow
question_answer 252) In which one of the following preparations, are you likely to come across cell junctions most frequently?
A)
Ciliated epithelium
done
clear
B)
Thrombocytes
done
clear
C)
Tendon
done
clear
D)
Hyaline cartilage
done
clear
View Answer play_arrow
question_answer 253) A person who is on a long hunger strike and is surviving only on water, will have
A)
more sodium in his urine
done
clear
B)
less amino acids in his urine
done
clear
C)
more glucose in his blood
done
clear
D)
less urea in his urine
done
clear
View Answer play_arrow
question_answer 254) Which pair of the following belongs to Basidiomycetes?
A)
Birds nest fungi and puff balls
done
clear
B)
Puffballs and Claviceps
done
clear
C)
Peziza and stink horns
done
clear
D)
Morchella and mushrooms
done
clear
View Answer play_arrow
question_answer 255) Spore dissemination in some liverworts is aided by
A)
elaters
done
clear
B)
indusium
done
clear
C)
calyptra
done
clear
D)
peristome teeth
done
clear
View Answer play_arrow
question_answer 256) Which one of the following human activities would cause least damage to the ecosystem?
A)
Organic farming
done
clear
B)
Shirting agriculture
done
clear
C)
Urban expansion
done
clear
D)
Cultivation of gentically engineered plants
done
clear
View Answer play_arrow
question_answer 257) The Okazaki fragments in DNA chain growth
A)
result in transcription
done
clear
B)
polymerize in the 3 to 5 direction and forms replication fork
done
clear
C)
prove semi-conservative nature of DNA replication
done
clear
D)
polymerize in the 5 to 3 direction and explain 3 to 5 DNA replication
done
clear
View Answer play_arrow
question_answer 258) Opening of floral buds into flowers is a type of
A)
autonomic movement of locomotion
done
clear
B)
autonomic movement of variation
done
clear
C)
paratonic movement of growth
done
clear
D)
autonomic movement of growth
done
clear
View Answer play_arrow
question_answer 259) One gene-one enzyme relationship was established for the first time in
A)
Neurospora crassa
done
clear
B)
Salmonella typhimurium
done
clear
C)
Escherichia coli
done
clear
D)
Diplococcus pneumonia
done
clear
View Answer play_arrow
question_answer 260) One of the important consequences of geographical isolation is
A)
no change in the isolated fauna
done
clear
B)
preventing speciation
done
clear
C)
speciation through reproductive isolation
done
clear
D)
random creation of new species
done
clear
View Answer play_arrow
question_answer 261) The first acceptor of electrons from an excited chlorophyll molecule of photosystem-II is
A)
cytochrome
done
clear
B)
iron-sulphur protein
done
clear
C)
ferredoxin
done
clear
D)
quinine
done
clear
View Answer play_arrow
question_answer 262) Which of the following ecosystem type has the highest annual net primary productivity?
A)
Tropical rainforest
done
clear
B)
Tropical deciduous forest
done
clear
C)
Temperate evergreen forest
done
clear
D)
Temperate deciduous forest
done
clear
View Answer play_arrow
question_answer 263) Lysozyme that is present in perspiration, saliva and tears, destroys
A)
certain fungi
done
clear
B)
certain types of bacteria
done
clear
C)
all viruses
done
clear
D)
most virus-infected cells
done
clear
View Answer play_arrow
question_answer 264) Inheritance of skin colour in humans is an example of
A)
chromosomal aberration
done
clear
B)
point mutation
done
clear
C)
polygenic inheritance
done
clear
D)
codominance
done
clear
View Answer play_arrow
question_answer 265) In the leaves of \[{{C}_{4}}\]-plants, malic acid formation during \[C{{O}_{2}}\] fixation occurs in the cells of
A)
mesophyll
done
clear
B)
bundle sheath
done
clear
C)
phloem
done
clear
D)
epidermis
done
clear
View Answer play_arrow
question_answer 266) Which of the following is a slime mould?
A)
Rhizopus
done
clear
B)
Physarum
done
clear
C)
Thiobadllus
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
question_answer 267) Which one of the following is not a constituent of cell membrane?
A)
Cholesterol
done
clear
B)
Glycolipids
done
clear
C)
Proline
done
clear
D)
Phospholipids
done
clear
View Answer play_arrow
question_answer 268) The concept of chemical evolution is based on
A)
crystallization of chemicals
done
clear
B)
interaction of water, air and clay under intense heat
done
clear
C)
effect of solar radiation on chemicals
done
clear
D)
possible origin of life by combination of chemicals under suitable environmental conditions
done
clear
View Answer play_arrow
question_answer 269) Which part of ovary in mammals acts as an endocrine gland after ovulation?
A)
Graafian follicle
done
clear
B)
Stroma
done
clear
C)
Germinal epithelium
done
clear
D)
Vitelline membrane
done
clear
View Answer play_arrow
question_answer 270) If you suspect major deficiency of antibodies in a person, to which of the following would you look for confirmatory evidence?
A)
Serum albumins
done
clear
B)
Serum globulins
done
clear
C)
Fibrinogen in the plasma
done
clear
D)
Haemocytes
done
clear
View Answer play_arrow
question_answer 271) In the prothallus of a vascular cryptogam, the antherozoids and eggs mature at different times. As a result
A)
there is no change in success rate of fertilization
done
clear
B)
there is high degree of sterility
done
clear
C)
one can conclude that the plant is apomictic
done
clear
D)
Self-fertilization is prevented
done
clear
View Answer play_arrow
question_answer 272) In gymnosperms, the pollen chamber represents
A)
a cell in the pollen grain in which the sperms are formed
done
clear
B)
a cavity in the ovule in which pollen grains are stored after pollination
done
clear
C)
an opening in the megagametophyte through which the pollen tube approaches the egg
done
clear
D)
the microsporangium in which pollen grains develop
done
clear
View Answer play_arrow
question_answer 273) One of endangered species of Indian medicinal plants is that of
A)
Podophyllum
done
clear
B)
Ocimum
done
clear
C)
Garlic
done
clear
D)
Nepenthes
done
clear
View Answer play_arrow
question_answer 274) In the hexaploid wheat, the haploid (n) and basic (x) numbers of chromosomes are
A)
n = 7 and x = 21
done
clear
B)
n = 21 and x = 21
done
clear
C)
n = 21 and x = 14
done
clear
D)
n = 21 and x = 7
done
clear
View Answer play_arrow
question_answer 275) Which one of the following is a viral disease of poultry?
A)
Salmonellosis
done
clear
B)
Coryza
done
clear
C)
New castle disease
done
clear
D)
Pasteurellosis
done
clear
View Answer play_arrow
question_answer 276) During transcription, RNA polymerase holoenzyme binds to a gene promoter and assumes a saddle-like structure. What is its DNA-binding sequence?
A)
TTAA
done
clear
B)
AATT
done
clear
C)
CACC
done
clear
D)
TATA
done
clear
View Answer play_arrow
question_answer 277) In maize, hybrid vigour is exploited by
A)
bombarding the seeds with DNA
done
clear
B)
crossing of two inbred parental lines
done
clear
C)
harvesting seeds from the most productive plants
done
clear
D)
inducing mutations
done
clear
View Answer play_arrow
question_answer 278) What is true about Nereis, scorpion, cockroach and silver fish?
A)
They all have jointed paired appendages
done
clear
B)
They all possess dorsal heart
done
clear
C)
None of them is aquatic
done
clear
D)
They all belong to the same phylum
done
clear
View Answer play_arrow
question_answer 279) Ergot of rye is caused by a species of
A)
Phycophthora
done
clear
B)
Uncinula
done
clear
C)
Ustilago
done
clear
D)
Claviceps
done
clear
View Answer play_arrow
question_answer 280) Among the human ancestors, the brain size was more than 1000 cc in
A)
Homo neanderthalensis
done
clear
B)
Homo erectus
done
clear
C)
Ramapichecus
done
clear
D)
Homo habilis
done
clear
View Answer play_arrow
question_answer 281) The wavelength of light absorbed by \[{{P}_{r}}\] form of phytochrome is
A)
640 nm
done
clear
B)
680 nm
done
clear
C)
720 nm
done
clear
D)
620 nm
done
clear
View Answer play_arrow
question_answer 282) A person is having problems with calcium and phosphorus metabolism in his body. Which one of the following glands may not be functioning properly?
A)
Parathyroid
done
clear
B)
Parotid
done
clear
C)
Pancreas
done
clear
D)
Thyroid
done
clear
View Answer play_arrow
question_answer 283) Which one of the following is being utilized as a source of bio-diesel in the Indian countryside?
A)
Euphorbia
done
clear
B)
Beetroot
done
clear
C)
Sugarcane
done
clear
D)
Pongamia
done
clear
View Answer play_arrow
question_answer 284) Foolish seedling disease of rice led to the discovery of
A)
GA
done
clear
B)
ABA
done
clear
C)
2, 4-D
done
clear
D)
IAA
done
clear
View Answer play_arrow
question_answer 285)
Consider the following four statements (I-IV) about certain desert animals such as kangaroo rat. I. They have dark colour high rate of reproduction and excrete solid urine. II. They do not drink water, breathe at a slow rate to conserve water and have their body covered with thick hairs. III. They feed on dry seeds and do not require drinking water. IV. They excrete very concentrated urine and do not use water to regulate body temperature
Which two of the above statements for such animals are true?
A)
III and IV
done
clear
B)
II and III
done
clear
C)
III and I
done
clear
D)
I and II
done
clear
View Answer play_arrow
question_answer 286) In DNA molecule
A)
the total amount of purine nucleotides and pyrimidine nucleotides is not always equal
done
clear
B)
there are two strands, which run parallel in the \[5\to 3\] direction
done
clear
C)
the proportion of adenine in relation to thymine varies with the organism
done
clear
D)
there are two strands, which run antiparallel-one in \[5\to 3\] direction and other in \[3\to 5\]
done
clear
View Answer play_arrow
question_answer 287) Which one of the following is incorrect about the characteristics of protobionts (coacervates and microspheres) as envisaged in the abiogenic origin of life?
A)
They were able to reproduce
done
clear
B)
They could separate combinations of molecules from the surroundings
done
clear
C)
They were partially isolated from the surroundings
done
clear
D)
They could maintain an internal environment
done
clear
View Answer play_arrow
question_answer 288) Which one of the following pair of items correctly belongs to the category of organs mentioned against it?
A)
Thorns of-Analogous organs Bougainvillea and tendrils of Cucurbita
done
clear
B)
Nictitating-Vestigial organs membrane and blind spot in human eye
done
clear
C)
Nephridia of- Excretory organs earthworm and Malpighian tubules of cockroach
done
clear
D)
Wings of honey bee-Homologous organs and wings of crow
done
clear
View Answer play_arrow
question_answer 289) Which one of the following phyla is correctly matched with its two general characteristics?
A)
Arthropoda-Body divided into head, thorax and abdomen and respiration by tracheae
done
clear
B)
Chordata-Notochord at some stage and separate anal and urinary openings to the outside
done
clear
C)
Echinoder-Pentamerous radial mata symmetry and mostly internal fertilization
done
clear
D)
Mollusca - Normally oviparous and development through a trochophore or veliger larva
done
clear
View Answer play_arrow
question_answer 290) Wobble nypothesis was given by
A)
FHC Crick
done
clear
B)
Nirenberg
done
clear
C)
Holley
done
clear
D)
Khurana
done
clear
View Answer play_arrow
question_answer 291) Ascaris is characterized by
A)
absence of true coelom but presence of metamerism
done
clear
B)
presence of neither true coelom nor metamerism
done
clear
C)
presence of true coelom but absence of metamerism
done
clear
D)
presence of true coelom and metamerism (metamerisation)
done
clear
View Answer play_arrow
question_answer 292)
A lake near a village suffered heavy mortality of fishes within a few days. Consider the following reasons for this. I. Lots of urea and phosphate fertilizer were used in the crops in the vicinity. II. The area was sprayed with DDT by an aircraft. III. The lake water turned green and stinky. IV. Phytoplankton populations in the lake declined initially thereby greatly reducing photosynthesis.
Which two of the above were the main causes of fish mortality in the lake?
A)
II, III
done
clear
B)
III, IV
done
clear
C)
I, III
done
clear
D)
I, II
done
clear
View Answer play_arrow
question_answer 293) Trichoderma harzianum has proved a useful microorganism for
A)
bioremediation of contaminated soils
done
clear
B)
reclamation of wastelands
done
clear
C)
gene transfer in higher plants
done
clear
D)
biological control of soil-borne plant pathogens
done
clear
View Answer play_arrow
question_answer 294) Quercus species are the dominant component in
A)
temperate deciduous forests
done
clear
B)
alpine forests
done
clear
C)
scrub forests
done
clear
D)
tropical rain forests
done
clear
View Answer play_arrow
question_answer 295) A transgenic food crop, which may help in solving the problem of night blindness in developing countries is
A)
Flavr savr tomatoes
done
clear
B)
Starlink maize
done
clear
C)
Bt soybean
done
clear
D)
golden rice
done
clear
View Answer play_arrow
question_answer 296) Which one of the following pairs of plants structures has haploid number of chromosomes?
A)
Megaspore mother cell and antipodal cells
done
clear
B)
Egg cell and antipodal cells
done
clear
C)
Nucellus and antipodal cells
done
clear
D)
Egg nucleus and secondary nucleus
done
clear
View Answer play_arrow
question_answer 297) Gel electrophoresis is used for
A)
cutting of DNA into fragments
done
clear
B)
separation of DNA fragments according to their size
done
clear
C)
construction of recornbinant DNA by joining with cloning vectors
done
clear
D)
isolation of DNA molecule
done
clear
View Answer play_arrow
question_answer 298) Senescence as an active developmental cellular process in the growth and functioning of a flowering plant, is indicated in
A)
vessels and tracheid differentiation
done
clear
B)
leaf abscission
done
clear
C)
annual plants
done
clear
D)
floral parts
done
clear
View Answer play_arrow
question_answer 299) What will happen if the secretion of parietal cells of gastric gland is blocked with an inhibitor?
A)
Gastric juice will be deficient in chymosin
done
clear
B)
Gastric juice will be deficient in pepsinogen
done
clear
C)
In the absence of HCl secretion, inactive pepsinogen is not converted into the active enzyme pepsin
done
clear
D)
Enter kinase will not be released from the duodenal mucosa and so trypsinogen is not converted to trypsin
done
clear
View Answer play_arrow
question_answer 300) The length of different internodes in a culm of sugarcane is variable because of
A)
shoot apical meristem
done
clear
B)
position of axillary buds
done
clear
C)
size of leaf lamina at the node below each internode
done
clear
D)
intercalary meristem
done
clear
View Answer play_arrow