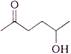
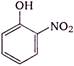
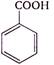
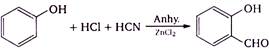question_answer 1) In refraction, light waves are bent on passing from one medium to the second medium, because in the second medium
A)
the frequency is different
done
clear
B)
the coefficient of elasticity is different
done
clear
C)
the speed is different
done
clear
D)
the amplitude is smaller
done
clear
View Answer play_arrow
question_answer 2) An engine pumps up 100 kg of water through a height of 10 m in 5 s. Given that, the efficiency of engine is 60% If \[g=10\,m{{s}^{-2}}\] the power of this engine is
A)
3.3 kW
done
clear
B)
0.33 kW
done
clear
C)
0.033 kW
done
clear
D)
33 kW
done
clear
View Answer play_arrow
question_answer 3) The angular amplitude of a simple pendulum is \[{{}_{0.}}\]The maximum-tension in its string will be
A)
\[\text{mg(1-}{{}_{0.}})\]
done
clear
B)
\[\text{mg}\,\text{(1+}{{}_{0.}})\]
done
clear
C)
\[\text{mg}\,\text{(1-}_{0}^{2})\]
done
clear
D)
\[\text{mg}\,\text{(1+}_{0}^{2})\]
done
clear
View Answer play_arrow
question_answer 4) During the melting of a slab of ice at 273 K at atmospheric pressure
A)
positive work is done by the ice-water system on the atmosphere
done
clear
B)
positive work is done on the ice-water system by the atmosphere
done
clear
C)
internal energy of ice-water system decreases
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 5) A solid sphere rolls down on two different inclined planes of same height, but of different inclinations. In both cases
A)
speed and time of descent will be same
done
clear
B)
speed will be same, but time of descent will be different
done
clear
C)
speed will be different, but time of descent will be same
done
clear
D)
speed and time of descent both are different
done
clear
View Answer play_arrow
question_answer 6) For inelastic collision between two spherical rigid bodies
A)
the total kinetic energy is conserved
done
clear
B)
the linear momentum is not conserved
done
clear
C)
the total mechanical energy is not conserved
done
clear
D)
the linear momentum is conserved
done
clear
View Answer play_arrow
question_answer 7) An object is kept on a smooth inclined plane of 1 in \[l.\] The horizontal acceleration to be imparted to the inclined plane so that the object is stationary relative to incline is
A)
\[g\sqrt{{{l}^{2}}-1}\]
done
clear
B)
\[g({{l}^{2}}-1)\]
done
clear
C)
\[\frac{g}{\sqrt{{{l}^{2}}-1}}\]
done
clear
D)
\[\frac{g}{{{l}^{2}}-1}\]
done
clear
View Answer play_arrow
question_answer 8) A point initially at rest moves along x-axis. Its acceleration varies with time as \[a=(6t+5)\,m/{{s}^{2}}\]. If it starts from origin, the distance covered in 2 s is
A)
20 m
done
clear
B)
18 m
done
clear
C)
16 m
done
clear
D)
25 m
done
clear
View Answer play_arrow
question_answer 9) A point source emits sound equally in all directions in a non-absorbing medium. Two points P and Q are at distances of 2 m and 3 m respectively from the source. The ratio of the intensities of the waves at P and Q is
A)
9 : 4
done
clear
B)
2 : 3
done
clear
C)
3 : 2
done
clear
D)
4 : 9
done
clear
View Answer play_arrow
question_answer 10) Which of the following expressions is that of a simple harmonic progressive wave?
A)
\[a\,\sin \,\omega t\]
done
clear
B)
\[a\,\sin \,(\omega t)\,\cos \,kx\]
done
clear
C)
\[a\,\sin \,(\omega t-kx)\,\]
done
clear
D)
\[a\cos kx\]
done
clear
View Answer play_arrow
question_answer 11) If \[E=\] energy, \[G=\] gravitational constant, \[I=\] impulse and \[M=\]mass, then dimensions of \[{{\frac{G\operatorname{I}M}{{{E}^{2}}}}^{2}}\]are same as that of
A)
time
done
clear
B)
mass
done
clear
C)
length
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 12) At what speed, the velocity head of water is equal to pressure head of 40 cm of Hg?
A)
10.3 m/s
done
clear
B)
2.8 m/s
done
clear
C)
5.6 m/s
done
clear
D)
8.4 m/s
done
clear
View Answer play_arrow
question_answer 13) An electric fan is switched on in a closed room. The air in the room is
A)
cooled
done
clear
B)
heated
done
clear
C)
maintains its temperature
done
clear
D)
heated or cooled depending on the atmospheric pressure
done
clear
View Answer play_arrow
question_answer 14) If the electric flux entering and leaving an enclosed surface respectively are \[{{\phi }_{1}}\] and \[{{\phi }_{2,}}\] the electric charge inside the surface will be
A)
\[\frac{{{\phi }_{2}}-{{\phi }_{1}}}{{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{{{\phi }_{2}}+{{\phi }_{1}}}{{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{{{\phi }_{1}}-{{\phi }_{2}}}{{{\varepsilon }_{0}}}\]
done
clear
D)
\[{{\varepsilon }_{0}}({{\phi }_{1}}+{{\phi }_{2}})\]
done
clear
View Answer play_arrow
question_answer 15) In the propagation of light waves, the angle between the direction of vibration and plane of polarization is
A)
\[0{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[80{}^\circ \]
done
clear
View Answer play_arrow
question_answer 16) How should people wearing spectacles work with a microscope?
A)
They should keep on wearing their spectacles
done
clear
B)
They should take off their spectacles
done
clear
C)
They may keep on wearing or take off their spectacles, it makes no difference
done
clear
D)
They cannot use a microscope at all
done
clear
View Answer play_arrow
question_answer 17) The root mean square and most probable speed of the molecules in a gas are
A)
same
done
clear
B)
different
done
clear
C)
cannot say
done
clear
D)
depends on nature of the gas
done
clear
View Answer play_arrow
question_answer 18) When you make ice cubes, the entropy of water
A)
does not change
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
may either increase or decrease depending on the process used
done
clear
View Answer play_arrow
question_answer 19) An electron and proton enter a magnetic Held perpendicularly. Both have same kinetic energy. Which of the following is true?
A)
Trajectory of electron is less curved
done
clear
B)
Trajectory of proton is less curved
done
clear
C)
Both trajectories are equally curved
done
clear
D)
Both move on straight line path
done
clear
View Answer play_arrow
question_answer 20) In an L-R circuit, time constant is that time in which current grows from zero to the value where \[{{I}_{0}}\] is steady state current.
A)
\[0.63{{I}_{0}}\]
done
clear
B)
\[0.50{{I}_{0}}\]
done
clear
C)
\[0.37{{I}_{0}}\]
done
clear
D)
\[{{I}_{0}}\]
done
clear
View Answer play_arrow
question_answer 21) The distance between two successive atomic planes of a calcite crystal is 0.3 nm. The minimum angle for Bragg scattering of \[0.3\,\overset{\text{o}}{\mathop{\text{A}}}\,\] X-rays will be
A)
\[1.43{}^\circ \]
done
clear
B)
\[1.56{}^\circ \]
done
clear
C)
\[2.86{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 22) A light emitting diode (I.ED) has a voltage drop of 2 V across it and passes a current of 10 n-I A. When it operates with a 6 V battery through a limiting resistor R, the value of R is
A)
40 \[k\,\Omega \]
done
clear
B)
\[4\,k\,\Omega \]
done
clear
C)
200 \[\,\Omega \]
done
clear
D)
400 \[\,\Omega \]
done
clear
View Answer play_arrow
question_answer 23) The minimum potential difference between the base and emitter required to switch a silicon transistor ON is approximately?
A)
1 V
done
clear
B)
3 V
done
clear
C)
5 V
done
clear
D)
4.2 V
done
clear
View Answer play_arrow
question_answer 24) Two balloons are filled, one with pure He gas and the other by air, respectively. If the pressure and temperature of these balloons are same then the number of molecules per unit volume is
A)
more in the He filled balloon
done
clear
B)
same in both balloons
done
clear
C)
more in air filled balloon
done
clear
D)
in the ratio of 1 : 4
done
clear
View Answer play_arrow
question_answer 25) A spaceman in training is rotated in a seat at the end of a horizontal arm of length 5 m- If he can with is and acceleration up to 9g. then what is the maximum number of revolutions per second permissible ? (Take g =10 m/s )
A)
13.5 rev/s
done
clear
B)
1.35 rev/s
done
clear
C)
0.675 rev/s
done
clear
D)
6.75 rev/s
done
clear
View Answer play_arrow
question_answer 26) Two stones are projected with the same speed but making different angles with the horizontal. Their horizontal ranges are equal. The angle of projection of one is \[\frac{\pi }{3}\]and the maximum height reached by it is 102 m. Then the maximum height reached by the other in metre is
A)
336
done
clear
B)
224
done
clear
C)
56
done
clear
D)
34
done
clear
View Answer play_arrow
question_answer 27) A cosmonaut is orbiting earth in a spacecraft at an altitude h = 630 km with a speed of 8 km/s. If the radius of the earth is 6400 km, the acceleration of the cosmonaut is
A)
\[9.10\,m/{{s}^{2}}\]
done
clear
B)
\[9.80\,m/{{s}^{2}}\]
done
clear
C)
\[10.0\,m/{{s}^{2}}\]
done
clear
D)
\[9.88\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 28) For two resistance wires joined in parallel, the resultant resistance is \[\frac{6}{5}\Omega .\] When one of the resistance wires breaks the effective resistance becomes \[2\,\Omega \] The resistance of the broken wire is
A)
\[\frac{3}{5}\,\Omega \]
done
clear
B)
\[2\,\Omega \]
done
clear
C)
\[\frac{6}{5}\,\Omega \]
done
clear
D)
\[3\,\Omega \]
done
clear
View Answer play_arrow
question_answer 29) When a p-n junction diode is reverse biased, then
A)
no current flows
done
clear
B)
the depletion region is increased
done
clear
C)
the depletion region is reduced
done
clear
D)
the height of the potential barrier is reduced
done
clear
View Answer play_arrow
question_answer 30) The operation of a nuclear reactor is said to be critical, if the multiplication factor (k) has a value
A)
1
done
clear
B)
1.5
done
clear
C)
2.1
done
clear
D)
2.5
done
clear
View Answer play_arrow
question_answer 31) Which one of the following is a possible nuclear reaction?
A)
\[_{5}^{10}B+_{2}^{4}He\xrightarrow{{}}_{7}^{13}N+_{1}^{1}H\]
done
clear
B)
\[_{11}^{23}Na+_{1}^{1}H\xrightarrow{{}}_{10}^{20}Ne+_{2}^{4}He\]
done
clear
C)
\[_{93}^{239}Np\xrightarrow{{}}_{94}^{239}Pu+\text{-+}\overset{-}{\mathop{v}}\,\]
done
clear
D)
\[_{7}^{11}N_{1}^{1}H\xrightarrow{{}}_{6}^{12}C+\,{{}^{-}}+v\]
done
clear
View Answer play_arrow
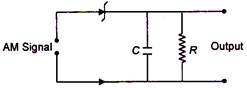
question_answer 32)
Given below is a circuit diagram of an AM demodulator. For good demodulation of AM signal of carrier frequency\[f,\]the value of RC should be
A)
\[RC=\frac{1}{f}\]
done
clear
B)
\[RC<\frac{1}{f}\]
done
clear
C)
\[RC\ge \frac{1}{f}\]
done
clear
D)
\[RC>>\frac{1}{f}\]
done
clear
View Answer play_arrow
question_answer 33) The ionization energy of 10 times ionized sodium atom is
A)
\[\frac{13.6}{11}eV\]
done
clear
B)
\[\frac{13.6}{{{(11)}^{2}}}eV\]
done
clear
C)
\[13.6\times {{(11)}^{2}}eV\]
done
clear
D)
\[13.6\,eV\]
done
clear
View Answer play_arrow
question_answer 34) \[n\,\text{-}\]particles per second are being emitted by N atoms of a radioactive element. The half-life of element will be
A)
\[\left( \frac{n}{N}s \right)\]
done
clear
B)
\[\left( \frac{N}{n} \right)s\]
done
clear
C)
\[\frac{0.693N}{n}s\]
done
clear
D)
\[\frac{0.693n}{N}s\]
done
clear
View Answer play_arrow
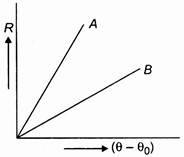
question_answer 35)
Two circular discs A and B with equal radii are blackened. They are heated to same temperature and are cooled under identical conditions. What inference do you draw from their cooling curves?
A)
A and B have .same specific heats
done
clear
B)
Specific heat of A is less
done
clear
C)
Specific heat of B is less
done
clear
D)
Nothing can be said
done
clear
View Answer play_arrow
question_answer 36) During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio\[\frac{{{C}_{p}}}{{{C}_{V}}}\] for the gas is
A)
\[\frac{4}{3}\]
done
clear
B)
\[2\]
done
clear
C)
\[\frac{3}{2}\]
done
clear
D)
\[\frac{3}{2}\]
done
clear
View Answer play_arrow
question_answer 37) A microscope is of cussed on a mark on a piece of paper and then a slab of glass of thickness 3 cm and refractive index 1.5 is placed over the mark. How should the microscope be moved to get the mark again in focus?
A)
2 cm upward
done
clear
B)
1 cm upward
done
clear
C)
45 cm downward
done
clear
D)
1 cm downward
done
clear
View Answer play_arrow
question_answer 38) The sum of two vectors \[\mathbf{\vec{A}}\] and \[\mathbf{\vec{B}}\] is at right angle to their difference. Then
A)
A = B
done
clear
B)
A = 2B
done
clear
C)
B = 2A
done
clear
D)
\[\mathbf{\vec{A}}\]and \[\mathbf{\vec{B}}\] have the same direction
done
clear
View Answer play_arrow
question_answer 39) The height \[y\] and the distance \[x\] along the horizontal plane of a projectile on a certain planet (with no surrounding atmosphere) are given by y = (8t - 5t) metre and x =- 6t metre where r is in seconds. The velocity of projection is
A)
8 m/s
done
clear
B)
6 m/s
done
clear
C)
10 m/s
done
clear
D)
Not obtained from the data
done
clear
View Answer play_arrow
question_answer 40) A ball is thrown up at an angle with the horizontal. Then the total change of momentum by the instant it returns to ground is
A)
acceleration due to gravity x total time of night
done
clear
B)
weight of the ball x half the time of flight
done
clear
C)
weight of the ball x total time of flight
done
clear
D)
weight of the ball x horizontal range
done
clear
View Answer play_arrow
question_answer 41) When a spring is stretched by a distance x, it exerts a force, given by \[F=(5x-16{{x}^{3}})N\]The work done, when the spring is stretched from 0.1 m to 0.2 m is
A)
\[8.7\times {{10}^{-2}}\text{J}\]
done
clear
B)
\[12.2\times {{10}^{-2}}\text{J}\]
done
clear
C)
\[8.7\times {{10}^{-4}}\text{J}\]
done
clear
D)
\[12.2\times {{10}^{-1}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 42) A hole is in the bottom of the tank having water. If total pressure at the bottom is 3 atm \[(1\,\text{atm=1}{{\text{0}}^{5}}\,N{{m}^{-2}}),\] then velocity of water flowing from hole is
A)
\[\sqrt{400}\,m{{s}^{-1}}\]
done
clear
B)
\[\sqrt{600}\,m{{s}^{-1}}\]
done
clear
C)
\[\sqrt{60}\,m{{s}^{-1}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 43) Time period of a simple pendulum of length (is \[{{T}_{1}}\]and time period of a uniform rod of the same length \[l\] pivoted about one end and oscillating in a vertical plane is \[{{T}_{2.}}\] Amplitude of oscillations in both the cases is small. Then \[\frac{{{T}_{1}}}{{{T}_{2}}}\] is
A)
\[\frac{1}{\sqrt{3}}\]
done
clear
B)
\[1\]
done
clear
C)
\[\sqrt{\frac{4}{3}}\]
done
clear
D)
\[\sqrt{\frac{3}{2}}\]
done
clear
View Answer play_arrow
question_answer 44)
An insulator plate is passed between the plates of a capacitor. Then current
A)
first flows from A to Band then from B to A
done
clear
B)
first flows from B to A and then from A to B
done
clear
C)
always flows from B to A
done
clear
D)
always flows from A to B
done
clear
View Answer play_arrow
question_answer 45) Two parallel large thin metal sheets have equal surface charge densities \[(\sigma =26.4\times {{10}^{-12}}\,c/{{m}^{2}})\] of opposite signs. The electric field between these sheets is
A)
\[\text{1}\text{.5}\,\text{N/C}\]
done
clear
B)
\[\text{1}\text{.5 }\!\!\times\!\!\text{ }\,\text{1}{{\text{0}}^{\text{-10}}}\,\text{N/C}\]
done
clear
C)
\[3\,\text{N/C}\]
done
clear
D)
\[\text{3 }\!\!\times\!\!\text{ }\,\text{1}{{\text{0}}^{\text{-10}}}\text{N/C}\]
done
clear
View Answer play_arrow
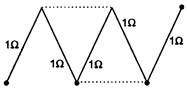
question_answer 46)
A circuit consists of five identical conductors as shown in figure. The two similar conductors are added as indicated by dotted lines. The ratio of resistances before and after addition will be
A)
\[\frac{7}{5}\]
done
clear
B)
\[\frac{3}{5}\]
done
clear
C)
\[\frac{5}{3}\]
done
clear
D)
\[\frac{6}{5}\]
done
clear
View Answer play_arrow
question_answer 47) The electric current passes through a metallic wire produces heat because of
A)
collisions of conduction electrons with each other
done
clear
B)
collisions of the atoms of the metal with each other
done
clear
C)
the energy released in the ionization of the atoms of the metal
done
clear
D)
collisions of the conduction electrons with the atoms of the metallic wire
done
clear
View Answer play_arrow
question_answer 48) Two concentric circular coils of ten turns each are situated in the same plane. Their radii are 20 cm and 40 cm and they carry respectively 0.2A and 0.3A currents in opposite direction. The magnetic field in tesla at the centre is
A)
\[\text{35}{{}_{\text{0}}}\text{/4}\]
done
clear
B)
\[{{}_{\text{0}}}\text{/80}\]
done
clear
C)
\[\text{7}{{}_{\text{0}}}\text{/80}\]
done
clear
D)
\[\text{5}{{}_{\text{0}}}\text{/4}\]
done
clear
View Answer play_arrow
question_answer 49) Electromagnetic waves with frequencies greater than the critical frequency of ionosphere cannot be used for communication using sky wave propagation, because
A)
the refractive index of the ionosphere becomes very high for \[f>{{f}_{c}}\]
done
clear
B)
the refractive index of the ionosphere becomes very low for \[f>{{f}_{c}}\]
done
clear
C)
the refractive index of the ionosphere becomes very high for \[f<{{f}_{c}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 50) In a choke coil, the reactance\[{{X}_{L}}\]and resistance R are such that
A)
\[{{X}_{L}}=R\]
done
clear
B)
\[{{X}_{L}}>>R\]
done
clear
C)
\[{{X}_{L}}<<R\]
done
clear
D)
\[{{X}_{L}}\infty \]
done
clear
View Answer play_arrow
question_answer 51) If C and R denote capacity and resistance, the dimensions of CR are
A)
\[[{{M}^{0}}{{L}^{0}}T]\]
done
clear
B)
\[[M{{L}^{0}}T]\]
done
clear
C)
\[[{{M}^{0}}{{L}^{0}}{{T}^{2}}]\]
done
clear
D)
not expressible in terms of M, L and T
done
clear
View Answer play_arrow
question_answer 52) An arrow is shot into air. Its range is 200 m and its time of flight is 5 s. If \[g=10\,m/{{s}^{2}},\] then the horizontal component of velocity of the arrow is
A)
12.5 m/s
done
clear
B)
25 m/s
done
clear
C)
31.25 m/s
done
clear
D)
40 m/s
done
clear
View Answer play_arrow
question_answer 53) A force of 49 N is just able to move a block of wood weighing 10 kg on a rough horizontal surface. Its coefficient of friction is
A)
\[1\]
done
clear
B)
0.7
done
clear
C)
0.5
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 54) Two bodies of masses m and 2m have equal kinetic energies. The ratio of their linear momenta is
A)
\[1\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 55) A ball of mass m elastically collides with a wall with velocity v, the n change in its momentum is equal to
A)
2m
done
clear
B)
2mv
done
clear
C)
8 mv
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 56) A solid sphere is rotating about a diameter at an angular velocity \[\omega .\] If it coots so that its radius reduces to \[\frac{1}{n}\] of its original value, its angular velocity becomes
A)
\[\frac{\omega }{n}\]
done
clear
B)
\[\frac{\omega }{{{n}^{2}}}\]
done
clear
C)
\[n\omega \]
done
clear
D)
\[{{n}^{2}}\omega \]
done
clear
View Answer play_arrow
question_answer 57) Two satellites A and B go around a planet P in circular orbits having radius 4R and R respectively. If the speed of satellite A is 3v, then the speed of satellite B will be
A)
6 v
done
clear
B)
9 v
done
clear
C)
3 v
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 58) When a drop splits up into a number of drops, then
A)
area decreases
done
clear
B)
volume increases
done
clear
C)
energy is absorbed
done
clear
D)
energy is liberated
done
clear
View Answer play_arrow
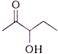
question_answer 59) A 5 m aluminium wire \[\text{(Y=7 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{10}}}\text{N/}{{\text{m}}^{\text{2}}}\text{)}\] of diameter 3 mm supports a 40 kg mass. In order to have the same elongation in a copper wire \[\text{(Y=12 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{10}}}\text{N/}{{\text{m}}^{\text{2}}}\text{)}\] of the same length under the same weight, the diameter should be in mm
A)
1.75
done
clear
B)
2.0
done
clear
C)
2.3
done
clear
D)
5.0
done
clear
View Answer play_arrow
question_answer 60) At which of the following temperatures, the value of surface tension of water is minimum?
A)
\[4{}^\circ C\]
done
clear
B)
\[25{}^\circ C\]
done
clear
C)
\[50{}^\circ C\]
done
clear
D)
\[75{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 61) In isochoric process
A)
\[\Delta W=0\]
done
clear
B)
\[\Delta U=0\]
done
clear
C)
\[\Delta Q=0\]
done
clear
D)
\[\Delta T=0\]
done
clear
View Answer play_arrow
question_answer 62) If the temperature difference on the two sides of a wall increases from \[100{}^\circ C\] to \[200{}^\circ C\], its thermal conductivity
A)
remains unchanged
done
clear
B)
is doubled
done
clear
C)
is halved
done
clear
D)
becomes four times
done
clear
View Answer play_arrow
question_answer 63) Let there be four articles having colors blue, red, black and white. When they are heated together and allowed to cool, which article will cool at the earliest?
A)
Blue
done
clear
B)
Red
done
clear
C)
Black
done
clear
D)
White
done
clear
View Answer play_arrow
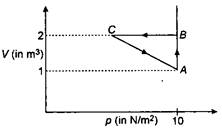
question_answer 64)
An ideal gas is taken through the cycle \[A\to B\to C\to A,\] as shown in figure. If the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process. \[C\to A\] is
A)
- 5 J
done
clear
B)
- 10 J
done
clear
C)
-15 J
done
clear
D)
-20 J
done
clear
View Answer play_arrow
question_answer 65) A piece of red glass when heated in dark to red hot state will appear to be
A)
white
done
clear
B)
red
done
clear
C)
green
done
clear
D)
invisible
done
clear
View Answer play_arrow
question_answer 66) The period of oscillation of a simple pendulum of constant length al surface of the earth is T. Its time period inside a mine will be
A)
cannot be compared
done
clear
B)
equal to T
done
clear
C)
less than T
done
clear
D)
more than T
done
clear
View Answer play_arrow
question_answer 67) A particle executing simple harmonic motion has a time period of 4s. After how much interval of time from t = 0 will its displacement be half of its amplitude ?
A)
\[\frac{1}{3}s\]
done
clear
B)
\[\frac{1}{2}s\]
done
clear
C)
\[\frac{2}{3}s\]
done
clear
D)
\[\frac{1}{6}s\]
done
clear
View Answer play_arrow
question_answer 68) A train approaches a stationary observer, the velocity of train being \[\frac{1}{20}\] of the velocity of sound. A sharp blast is blown with the whistle of the engine at equal intervals of a second. The interval between the successive blasts as heard by the observer is
A)
\[\frac{1}{20}s\]
done
clear
B)
\[\frac{1}{20}\min \]
done
clear
C)
\[\frac{19}{20}s\]
done
clear
D)
\[\frac{19}{20}\min \]
done
clear
View Answer play_arrow
question_answer 69) Which of the following equations represents a wave travelling along y-axis ?
A)
\[y=A\,\sin \,(kx-\omega t)\]
done
clear
B)
\[x=A\,\sin \,(ky-\omega t)\]
done
clear
C)
\[y=A\,\sin \,(ky\cos \omega t\]
done
clear
D)
\[y=A\,\,\cos ky\sin \omega t\]
done
clear
View Answer play_arrow
question_answer 70) The difference between soft and hard X-rays is of
A)
velocity
done
clear
B)
intensity
done
clear
C)
frequency
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 71) The capacity of an isolated conducting sphere of radius R is proportional to
A)
\[{{R}^{2}}\]
done
clear
B)
\[\frac{1}{{{R}^{2}}}\]
done
clear
C)
\[\frac{1}{R}\]
done
clear
D)
\[R\]
done
clear
View Answer play_arrow
question_answer 72) Two spheres of radii \[{{R}_{1}}\] and \[{{R}_{2}}\] respectively are charged and joined by a wire. The ratio of electric fields of spheres is
A)
\[\frac{R\,_{2}^{2}}{R_{1}^{2}}\]
done
clear
B)
\[\frac{R\,_{1}^{2}}{R_{2}^{2}}\]
done
clear
C)
\[\frac{R{{\,}_{2}}}{{{R}_{1}}}\]
done
clear
D)
\[\frac{R{{\,}_{1}}}{{{R}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 73) If a positive charge is shifted from a low potential region to a high potential region, then electric potential energy
A)
decreases
done
clear
B)
increases
done
clear
C)
remains the same
done
clear
D)
may increase or decrease
done
clear
View Answer play_arrow
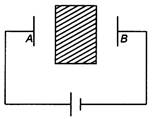
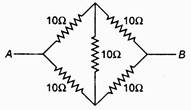
question_answer 74)
The equivalent resistance between A and B is
A)
\[10\,\Omega \]
done
clear
B)
\[20\,\Omega \]
done
clear
C)
\[30\,\Omega \]
done
clear
D)
\[40\,\Omega \]
done
clear
View Answer play_arrow
question_answer 75) An electrical cable of copper has just one wire of radius 9 mm. Its resistance is \[5\,\Omega \] The single, wire of the cable is replaced by 6 different well insulated copper wires each of radius 3 mm. The total resistance of the cable will now be equal to
A)
\[270\,\Omega \]
done
clear
B)
\[90\,\Omega \]
done
clear
C)
\[45\,\Omega \]
done
clear
D)
\[7.5\,\Omega \]
done
clear
View Answer play_arrow
question_answer 76) A current of 2 A flowing through a conductor produces 80 Jot heat in 10s. The resistances of the conductor is
A)
\[0.5\,\Omega \]
done
clear
B)
\[2\,\Omega \]
done
clear
C)
\[4\,\Omega \]
done
clear
D)
\[20\,\Omega \]
done
clear
View Answer play_arrow
question_answer 77) A thermoelectric refrigerator works on
A)
Joule effect
done
clear
B)
Seebeck effect
done
clear
C)
Peltier effect
done
clear
D)
Thermionic effect
done
clear
View Answer play_arrow
question_answer 78) A small circular flexible loop of wire of radius. \[r\] carries a current\[I.\]It is placed in a uniform magnetic field B. The tension in the loop will be doubled if
A)
\[I\] is halved
done
clear
B)
B is halved
done
clear
C)
r is doubled
done
clear
D)
both B and \[I\] are doubled
done
clear
View Answer play_arrow
question_answer 79) Two parallel wires carrying currents in the same direction attract each other because of
A)
potential difference between them
done
clear
B)
mutual inductance between them
done
clear
C)
electric force between them
done
clear
D)
magnetic force between them
done
clear
View Answer play_arrow
question_answer 80) A hydrogen atom is paramagnetic. A hydrogen molecule is
A)
diamagnetic
done
clear
B)
paramagnetic
done
clear
C)
ferromagnetic
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 81) A galvanometer has resistance of \[400\,\Omega \] am deflects full scale for current of 0.2mA through it. The shunt resistance required to convert into 3 A ammeter is
A)
\[0.027\,\Omega \]
done
clear
B)
\[0.054\,\Omega \]
done
clear
C)
\[0.0135\,\Omega \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 82) the power factor of a series L-C-R circuit when at resonance is
A)
zero
done
clear
B)
0.5
done
clear
C)
1.0
done
clear
D)
depends on values of L, C and R
done
clear
View Answer play_arrow
question_answer 83) For high frequency, capacitor offers
A)
more resistance
done
clear
B)
less resistance
done
clear
C)
zero resistance
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 84) In an oscillating L-C circuit, the maximum charge on the capacitor is Q. The charge on the capacitor, when the energy is stored equally between the electric and magnetic field is
A)
\[\frac{Q}{2}\]
done
clear
B)
\[\frac{Q}{\sqrt{2}}\]
done
clear
C)
\[\frac{Q}{\sqrt{3}}\]
done
clear
D)
\[\frac{Q}{3}\]
done
clear
View Answer play_arrow
question_answer 85) If\[{{v}_{g,}}\] \[{{v}_{x,}}\] and \[{{v}_{m,}}\] are the speeds of gamma rays, X-rays and microwaves respectively in vacuum then
A)
\[{{v}_{g,}}>{{v}_{m}}>{{v}_{x}}\]
done
clear
B)
\[{{v}_{g}}>{{v}_{x}}>{{v}_{m}}\]
done
clear
C)
\[{{v}_{g}}={{v}_{x}}={{v}_{m}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 86) A person standing in front of a mirror finds his image larger than himself. This implies that the mirror is
A)
convex
done
clear
B)
parabolic
done
clear
C)
plane
done
clear
D)
concave
done
clear
View Answer play_arrow
question_answer 87) For a telescope, larger the diameter of the objective lens
A)
greater is the resolving power
done
clear
B)
smaller is the resolving power
done
clear
C)
greater is the magnifying power
done
clear
D)
smaller is the magnifying power
done
clear
View Answer play_arrow
question_answer 88) If [he critical angle for total internal reflection from medium to vacuum is \[30{}^\circ \], the velocity of light in medium is
A)
\[3\times {{10}^{8}}\,m/s\]
done
clear
B)
\[1.5\times {{10}^{8}}\,m/s\]
done
clear
C)
\[6\times {{10}^{8}}\,m/s\]
done
clear
D)
\[\sqrt{3}\,\times {{10}^{8}}\,m/s\]
done
clear
View Answer play_arrow
question_answer 89) The deviation is maximum for which colour?
A)
Violet
done
clear
B)
Red
done
clear
C)
Blue
done
clear
D)
Green
done
clear
View Answer play_arrow
question_answer 90) In single slit diffraction pattern
A)
central fringe has negligible width than others
done
clear
B)
all fringes are of same width
done
clear
C)
central fringe does not exist
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 91) The relation between amplification factor \[(\mu ),\] plate resistance \[({{r}_{p}})\]and mutual conductance \[({{g}_{m}})\]of a triode valve is given by
A)
\[\mu ={{r}_{p}}\times {{g}_{m}}\]
done
clear
B)
\[{{r}_{p}}=\mu \times {{g}_{m}}\]
done
clear
C)
\[{{g}_{m}}=\mu \times {{r}_{p}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 92) A light of wavelength \[5000\overset{\text{o}}{\mathop{\text{A}}}\,\] falls on a sensitive plate with photoelectric work function 1.90 eV. Kinetic energy of the emitted photoelectrons will be (Given, \[(\text{Give}\,\text{n,}\,\text{h=6}\text{.62}\times \text{1}{{\text{0}}^{-34}}\text{J-s})\]
A)
0.1 eV
done
clear
B)
2 eV
done
clear
C)
0 58 eV
done
clear
D)
1.581 eV
done
clear
View Answer play_arrow
question_answer 93) An electron with kinetic energy 5 eV is incident on an H-atom in its ground state. The collision
A)
must be elastic
done
clear
B)
may be partially elastic
done
clear
C)
may be completely elastic
done
clear
D)
may be completely inelastic
done
clear
View Answer play_arrow
question_answer 94) The emission of electrons is possible by
A)
photoelectric effect
done
clear
B)
thermionic effect
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 95) An electron and a proton are possessing same amount of kinetic energies. The de-Broglie wavelength is greater for
A)
electron
done
clear
B)
proton
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 96) In a sample of radioactive -material, what percentage of the initial number of active nuclei will decay during one mean life?
A)
37%
done
clear
B)
50%
done
clear
C)
63%
done
clear
D)
69.3%
done
clear
View Answer play_arrow
question_answer 97) For the stability of any nucleus
A)
binding energy per nucleon will he more
done
clear
B)
binding energy per nucleon wilt be less
done
clear
C)
number of electrons will be more
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 98) If the half-life of any sample of radioactive substance is 4 days, then the fraction of sample will remain undecayed after 2 days, will be?
A)
\[\sqrt{2}\]
done
clear
B)
\[\frac{1}{\sqrt{2}}\]
done
clear
C)
\[\frac{\sqrt{2}-1}{\sqrt{2}}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 99) A sensitive magnetic instrument can be shielded very effectively from outside fields by placing it inside a box of
A)
teak wood
done
clear
B)
plastic material
done
clear
C)
soft iron of high permeability
done
clear
D)
a metal of high conductivity
done
clear
View Answer play_arrow
question_answer 100) An n-type semiconductor is
A)
negatively charged
done
clear
B)
positively charged
done
clear
C)
neutral
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 101) The compound in which underlined carbon uses only its \[s{{p}^{3}}\] hybrid orbitals for bond formation is
A)
\[C{{H}_{3}}\underline{C}OOH\]
done
clear
B)
\[C{{H}_{3}}\underline{C}ON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}\underline{C}{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}\underline{C}H=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 102) \[t-\]butyl alcohol is
A)
2-methyl-propan-2-ol
done
clear
B)
2-methyl-propan-1-ol
done
clear
C)
3-methyt-buian-1-ol
done
clear
D)
3-methyl-butan-2-ol
done
clear
View Answer play_arrow
question_answer 103) Optical isomerism is shown by
A)
propanol-2
done
clear
B)
butanol-2
done
clear
C)
ethanol
done
clear
D)
methanol
done
clear
View Answer play_arrow
question_answer 104) When\[{{C}_{2}}{{H}_{2}},\,\,C{{H}_{4}}\]and \[{{C}_{2}}{{H}_{4}}\] passes through a test tube which have ammoniacal \[C{{u}_{2}}C{{l}_{2}}\], find out which gas comes out unaffected from test tube?
A)
\[{{C}_{2}}{{H}_{2}}\]and\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]and\[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]and\[C{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 105) If a mixture of \[CO\] and \[{{N}_{3}}\] in equal amount have total \[1\,\,atm\] pressure, find out partial pressure of \[{{N}_{3}}\] in mixture
A)
\[1\,\,atm\]
done
clear
B)
\[0.50\,\,atm\]
done
clear
C)
\[2\,\,atm\]
done
clear
D)
\[3\,\,atm\]
done
clear
View Answer play_arrow
question_answer 106) Which does not reacts with Fehling solution?
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
Glucose
done
clear
View Answer play_arrow
question_answer 107) When hydrogen molecules decomposed into its atoms which conditions gives maximum yields of \[H\] atoms?
A)
High temperature and low pressure
done
clear
B)
Low temperature and high pressure
done
clear
C)
High temperature and high pressure
done
clear
D)
Low temperature and low pressure
done
clear
View Answer play_arrow
question_answer 108) Lithopone is
A)
\[ZnS{{O}_{4}}+PbS\]
done
clear
B)
\[BaS{{O}_{4}}+ZnS\]
done
clear
C)
\[Pb{{O}_{2}}\]
done
clear
D)
\[ZnS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 109) Natural rubber is a polymer of
A)
styrene
done
clear
B)
chloroprene
done
clear
C)
\[C{{H}_{2}}=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-CH=C{{H}_{2}}\]or isopropene
done
clear
D)
1, 3-butadiene
done
clear
View Answer play_arrow
question_answer 110) \[PC{{l}_{3}}\] is possible but \[NC{{l}_{5}}\] does not exist
A)
in \[N,\,\,d-\]sub-shell is absent
done
clear
B)
ionization energy of \[N\] is very high
done
clear
C)
it does not like\[Cl\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 111) \[_{11}N{{a}^{23}}\] is formed from \[_{11}N{{a}^{24}}\] by the
A)
\[_{0}{{n}^{1}}\] emission
done
clear
B)
\[\beta -\]emission
done
clear
C)
\[K-\]electron capture
done
clear
D)
\[\alpha -\]emission
done
clear
View Answer play_arrow
question_answer 112) Glucose reacts with excess of phenyl hydrazine and forms
A)
glucosazone
done
clear
B)
glucose phenyl hydrazine
done
clear
C)
glucose oxime
done
clear
D)
sorbitol
done
clear
View Answer play_arrow
question_answer 113) Which is purified by steam distillation?
A)
Aniline
done
clear
B)
Benzoic Acid
done
clear
C)
Petroleum
done
clear
D)
Naphthalene
done
clear
View Answer play_arrow
question_answer 114) Which compound is aromatic?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 115) Which does not replace hydrogen from\[HCl\]?
A)
\[Cu\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Na\]
done
clear
D)
\[Al\]
done
clear
View Answer play_arrow
question_answer 116) For prevention of rusting to iron, paints of which is used?
A)
\[PbO\]
done
clear
B)
\[Pb{{O}_{2}}\]
done
clear
C)
\[P{{b}_{3}}{{O}_{4}}\]
done
clear
D)
\[PbS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 117) A violet colour compound is formed in detection of \[S\] in a compound
A)
\[N{{a}_{4}}[Fe{{(CN)}_{5}}NOS]\]
done
clear
B)
\[N{{a}_{3}}[Fe{{(CN)}_{5}}NOS]\]
done
clear
C)
\[N{{a}_{2}}[Fe{{(CN)}_{5}}NOS]\]
done
clear
D)
\[N{{a}_{2}}[Fe{{(CN)}_{5}}NO]\]
done
clear
View Answer play_arrow
question_answer 118) Find out emf of cell, \[Zn;Z{{n}^{2+}}(1M)|\,|\,\,C{{u}^{2+}}(1M);\,\,Cu\,\,E{}^\circ \]for \[Z{{n}^{2+}}/Zn=-0.76;\,\,E{}^\circ for\,\,C{{u}^{2+}}/Cu=+0.34\]
A)
\[+1.10\,\,V\]
done
clear
B)
\[-1.10\,\,V\]
done
clear
C)
\[-0.76\]
done
clear
D)
\[-0.42\]
done
clear
View Answer play_arrow
question_answer 119) Radius of \[Ga\] is less than \[Al\] because of
A)
lanthenoid contraction
done
clear
B)
greater screening effect
done
clear
C)
inert pair effect
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 120) Number of atoms of \[He\] in \[100\,\,amu\] of \[He\] (atomic\[\text{wt}\text{.}\] of\[He\] is 4) are
A)
\[25\]
done
clear
B)
\[100\]
done
clear
C)
\[50\]
done
clear
D)
\[100\times 6\times {{10}^{-23}}\]
done
clear
View Answer play_arrow
question_answer 121) . Which inert gas show abnormal behaviour on liquidation?
A)
\[Xe\]
done
clear
B)
\[He\]
done
clear
C)
\[Ar\]
done
clear
D)
\[Kr\]
done
clear
View Answer play_arrow
question_answer 122) Which has least gold number?
A)
Gelatin
done
clear
B)
Starch
done
clear
C)
Albumin
done
clear
D)
Blood
done
clear
View Answer play_arrow
question_answer 123) In a compound \[C,\,\,H\] and \[N\] are present in \[9:13.5\] by weight. If molecular weight of the compound is\[108\], then the molecular formula on the compound is
A)
\[{{C}_{2}}{{H}_{6}}{{N}_{2}}\]
done
clear
B)
\[{{C}_{3}}{{H}_{4}}N\]
done
clear
C)
\[{{C}_{6}}{{H}_{8}}{{N}_{2}}\]
done
clear
D)
\[{{C}_{9}}{{H}_{12}}N\]
done
clear
View Answer play_arrow
question_answer 124) In the following reaction, \[{{C}_{2}}{{H}_{2}}\xrightarrow[HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}]{{{H}_{2}}O}XC{{H}_{3}}CHO\] What is\[X\]?
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}-O-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{2}}=CHOH\]
done
clear
View Answer play_arrow
question_answer 125) Which one of the following will most readily be dehydrated in acidic conditions?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 126) Indane is
A)
commercial butane, isobutane and propane mixture
done
clear
B)
butane, ethane mixture
done
clear
C)
commercial propane
done
clear
D)
methane, propane mixture
done
clear
View Answer play_arrow
question_answer 127) Which of the following is not Lewis acid?
A)
\[AlC{{l}_{3}}\cdot 6{{H}_{2}}O\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[SnC{{l}_{4}}\]
done
clear
D)
\[FeC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 128) Blue vitriol is
A)
\[MgS{{O}_{4}}\cdot 5{{H}_{2}}O\]
done
clear
B)
\[CuS{{O}_{4}}\cdot 5{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}\cdot 5{{H}_{2}}O\]
done
clear
D)
\[ZnS{{O}_{4}}\cdot 5{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 129) Bleaching action of\[CaOC{{l}_{2}}\]is due to
A)
nascent oxygen
done
clear
B)
chlorine
done
clear
C)
\[HClO\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 130) Which of the following is act as pickling agent?
A)
\[HN{{O}_{3}}\]
done
clear
B)
\[HCl\]
done
clear
C)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[HN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 131) In a closed insulated container, a liquid is stirred with a paddle to increase the temperature. Which of the following is true?
A)
\[\Delta E=W\ne 0,\,\,q=0\]
done
clear
B)
\[\Delta E=W=q\ne 0\]
done
clear
C)
\[\Delta E=W=q\ne 0\]
done
clear
D)
\[W=0,\,\,\Delta E=q\ne 0\]
done
clear
View Answer play_arrow
question_answer 132)
The following data are for the decomposition of ammonium nitrite in aqueous solution Vol. of N2 in cc Time (min.) 6.25 10 9.00 15 11.40 20 13.65 25 35.65 \[\infty \]
The order of reaction is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 133) In Arrhenius plot, intercepts is equal to
A)
\[\frac{-{{E}_{a}}}{R}\]
done
clear
B)
\[\ln A\]
done
clear
C)
\[\ln k\]
done
clear
D)
\[{{\log }_{10}}a\]
done
clear
View Answer play_arrow
question_answer 134) The total number of possible isomeric trimethyl benzene is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 135) An alkyl halide by formation of its Grignard reagent and heating with water yields propane. What is the original alkyl halide?
A)
Methyl iodide
done
clear
B)
Ethyl iodide
done
clear
C)
Ethyl bromide
done
clear
D)
Propyl bromide
done
clear
View Answer play_arrow
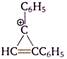
question_answer 136)
The following reaction is known as
A)
Perkin reaction
done
clear
B)
Gattermann reaction
done
clear
C)
Kolbe reaction
done
clear
D)
Gattermann-aldehyde reaction
done
clear
View Answer play_arrow
question_answer 137) Acetone on distillation with conc.\[{{H}_{2}}S{{O}_{4}}\] forms
A)
phorone
done
clear
B)
acrolein
done
clear
C)
mesitylene
done
clear
D)
mesityl oxide
done
clear
View Answer play_arrow
question_answer 138) On treatment with ninhydrin which of the following give blue colour?
A)
Proteins
done
clear
B)
Peptides
done
clear
C)
\[\alpha -\]amino acids
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 139) Aldehyde with \[N{{H}_{2}}\cdot N{{H}_{2}}\] forms
A)
hydrazones
done
clear
B)
aniline
done
clear
C)
nitrobenzene
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 140) Allyl isocyanide contains \[\sigma \] and \[\pi \] bonds
A)
\[9\sigma ,\,\,3\pi \]
done
clear
B)
\[9\sigma ,\,\,9\pi \]
done
clear
C)
\[3\sigma ,\,\,4\pi \]
done
clear
D)
\[5\sigma ,\,\,7\pi \]
done
clear
View Answer play_arrow
question_answer 141) vant Hoff factor of \[Ca{{(N{{O}_{3}})}_{2}}\] is
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 142) 1 mole of \[{{H}_{2}}\] and 2 moles of \[{{I}_{2}}\]are taken initially in \[a\,\,2\,\,I\]vessel. The number of moles of \[{{H}_{2}}\] at equilibrium is\[0.2\]. Then the number of moles of \[{{I}_{2}}\] and \[HI\] at equilibrium are
A)
\[1.2,\,\,1.6\]
done
clear
B)
\[1.8,\,\,1.0\]
done
clear
C)
\[0.4,\,\,2.4\]
done
clear
D)
\[0.8,\,\,2.0\]
done
clear
View Answer play_arrow
question_answer 143) Degree of dissociation of\[0.1\,\,N\,\,C{{H}_{3}}COOH\], is\[({{K}_{acid}}=1\times {{10}^{-5}})\]
A)
\[{{10}^{-5}}\]
done
clear
B)
\[{{10}^{-4}}\]
done
clear
C)
\[{{10}^{-3}}\]
done
clear
D)
\[{{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 144) \[3A\to 2B\]rate of reaction\[\frac{+d[B]}{dt}\]is equal to
A)
\[-\frac{3}{2}\frac{d[A]}{dt}\]
done
clear
B)
\[-\frac{2}{3}\frac{d[A]}{dt}\]
done
clear
C)
\[-\frac{1}{3}\frac{d[A]}{dt}\]
done
clear
D)
\[+2\frac{d[A]}{dt}\]
done
clear
View Answer play_arrow
question_answer 145) The volume of water to be added to \[100\,\,c{{m}^{3}}\] of \[0.5\,\,N\,\,{{H}_{2}}S{{O}_{4}}\] to get decinormal concentration is
A)
\[400\,\,c{{m}^{3}}\]
done
clear
B)
\[450\,\,c{{m}^{3}}\]
done
clear
C)
\[500\,\,c{{m}^{3}}\]
done
clear
D)
\[100\,\,c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 146) The compressibility of a gas is less than unity at\[STP\]. Therefore,
A)
\[{{V}_{m}}>22.4\,\,L\]
done
clear
B)
\[{{V}_{m}}<22.4\,\,L\]
done
clear
C)
\[{{V}_{m}}=22.4\,\,L\]
done
clear
D)
\[{{V}_{m}}=44.8\,\,L\]
done
clear
View Answer play_arrow
question_answer 147) \[1.520\,\,g\] of hydroxide of a metal on ignition gave \[0.995\,\,g\] of oxide. The equivalent weight of metal is
A)
\[1.52\]
done
clear
B)
\[0.995\]
done
clear
C)
\[190\]
done
clear
D)
\[9\]
done
clear
View Answer play_arrow
question_answer 148) Which of the following oxide of nitrogen is most thermally stable?
A)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
B)
\[{{N}_{2}}O\]
done
clear
C)
\[NO\]
done
clear
D)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 149) Which of the following gas is used in artificial respiration?
A)
\[{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
Helium
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 150) Which of the following gas is used in warfare?
A)
\[{{N}_{2}}O\]
done
clear
B)
\[CC{{l}_{3}}\cdot N{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 151) Essential compound to manufacture acrolein is
A)
glycerol
done
clear
B)
benzene
done
clear
C)
phenol
done
clear
D)
ammonia
done
clear
View Answer play_arrow
question_answer 152) Hybridization present in \[Cl{{F}_{3}}\] is
A)
\[s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[ds{{p}^{2}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
question_answer 153) Oxidation number of sulphur in \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] is
A)
\[+6\]
done
clear
B)
\[+8\]
done
clear
C)
\[+21\]
done
clear
D)
\[+7\]
done
clear
View Answer play_arrow
question_answer 154) Number of electrons surrounding \[Kr\] in\[Kr{{F}_{2}}\]is
A)
\[10\]
done
clear
B)
\[6\]
done
clear
C)
\[4\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 155) Reason of passivity of iron is
A)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
B)
\[F{{e}_{3}}{{O}_{4}}\]
done
clear
C)
\[FeO\]
done
clear
D)
\[F{{e}_{2}}{{O}_{4}}\cdot 3{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 156) Half-life of radioactive substance is 4 days. Amount of the substance decayed in two day is
A)
\[\frac{1}{\sqrt{2}}\]
done
clear
B)
\[\left( 1-\frac{1}{\sqrt{2}} \right){{N}_{0}}\]
done
clear
C)
\[20%\]
done
clear
D)
\[\frac{1}{8}\]
done
clear
View Answer play_arrow
question_answer 157) \[{{K}_{2}}Hg{{l}_{4}}\] is used to lest the following
A)
\[NH_{4}^{+}\]
done
clear
B)
\[Cl\]
done
clear
C)
\[Br\]
done
clear
D)
\[I\]
done
clear
View Answer play_arrow
question_answer 158) \[H-O-O\]bond angle in\[{{H}_{2}}{{O}_{2}}\]is
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{180}^{o}}\]
done
clear
C)
\[{{109.5}^{o}}\]
done
clear
D)
\[{{94.8}^{o}}\]
done
clear
View Answer play_arrow
question_answer 159) Iodine is tested by the following reagent
A)
starch
done
clear
B)
urea
done
clear
C)
glucose
done
clear
D)
glycerol
done
clear
View Answer play_arrow
question_answer 160) Half-life of a radioactive element is
A)
\[0.693\]
done
clear
B)
\[\frac{1}{0.693}\]
done
clear
C)
\[\frac{0.693}{k}\]
done
clear
D)
\[\frac{k}{0.693}\]
done
clear
View Answer play_arrow
question_answer 161) Radioactive metal is
A)
\[Li\]
done
clear
B)
\[Ce\]
done
clear
C)
\[Na\]
done
clear
D)
\[Ra\]
done
clear
View Answer play_arrow
question_answer 162) Which has smallest size?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[Ne\]
done
clear
D)
\[{{O}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 163) Phenol on reaction with \[B{{r}_{2}}\] gives
A)
\[o-\]bromophenol
done
clear
B)
\[p-\]bromophenol
done
clear
C)
\[2,\,\,4,\,\,6-\]tribromophenol
done
clear
D)
\[1,\,\,3,\,\,5-\]tribromobenzene
done
clear
View Answer play_arrow
question_answer 164) Electron deficient molecule is
A)
\[CC{{l}_{4}}\]
done
clear
B)
\[PC{{l}_{5}}\]
done
clear
C)
\[B{{F}_{3}}\]
done
clear
D)
\[S{{F}_{6}}\]
done
clear
View Answer play_arrow
question_answer 165) Which is the correct relation for diffusion a gases?
A)
\[r\propto d\]
done
clear
B)
\[r\propto \sqrt{d}\]
done
clear
C)
\[r\propto \frac{1}{\sqrt{d}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 166) Aniline with \[CHC{{l}_{3}}\] and \[KOH\] on heating gives
A)
isocyanide
done
clear
B)
cyanide
done
clear
C)
phenol
done
clear
D)
salicylic acid
done
clear
View Answer play_arrow
question_answer 167) Geometrical isomerism is found in
A)
pemene-1
done
clear
B)
propene
done
clear
C)
2-butene
done
clear
D)
butene-1
done
clear
View Answer play_arrow
question_answer 168) Most acidic is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 169) Hydrolysis of sodium acetate gives
A)
acidic solution
done
clear
B)
basic solution
done
clear
C)
amphoteric solution
done
clear
D)
neutral solution
done
clear
View Answer play_arrow
question_answer 170) Isotopic pair is
A)
\[_{20}{{X}^{40}},\,{{\,}_{21}}{{Y}^{40}}\]
done
clear
B)
\[_{20}{{X}^{40}},\,{{\,}_{20}}{{X}^{41}}\]
done
clear
C)
\[_{40}{{X}^{20}},\,{{\,}_{41}}{{X}^{20}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 171) \[Z\xrightarrow{PC{{l}_{5}}}X\xrightarrow{Alc.\,\,KOH}Y\xrightarrow[2{{H}_{2}}O/\Delta ]{conc.\,\,{{H}_{2}}S{{O}_{4}}}Z\] Here \[Z\] is
A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}-C-OH\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 172) Fuel, used in nuclear reactor is
A)
plutonium
done
clear
B)
thorium
done
clear
C)
radium
done
clear
D)
deuterium
done
clear
View Answer play_arrow
question_answer 173) Atomic number of an element is equal to the number of
A)
protons
done
clear
B)
neutrons
done
clear
C)
protons + electrons
done
clear
D)
protons + neutrons
done
clear
View Answer play_arrow
question_answer 174) Following pair is separated by yellow ammonium sulphide
A)
\[CdS,\,\,B{{i}_{2}}{{S}_{3}}\]
done
clear
B)
\[B{{i}_{2}}{{S}_{3}},\,\,PbS\]
done
clear
C)
\[PbS,\,\,HgS\]
done
clear
D)
\[CdS,\,\,A{{g}_{2}}{{S}_{3}}\]
done
clear
View Answer play_arrow
question_answer 175) Cationic hydrolysis gives the following solution
A)
acidic
done
clear
B)
basic
done
clear
C)
neutral
done
clear
D)
amphoteric
done
clear
View Answer play_arrow
question_answer 176) Which gives nucleophilic addition most easily?
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,HCHO\]
done
clear
D)
\[HCHO\]
done
clear
View Answer play_arrow
question_answer 177) Compound reacting with alkaline\[KMn{{O}_{4}}\]
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}C-C{{H}_{2}}OH\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 178) Lucas reagent reacts fastest with
A)
butanol-1
done
clear
B)
butanol-2
done
clear
C)
2-methyl-propanol-2
done
clear
D)
2-methyl-propanol-1
done
clear
View Answer play_arrow
question_answer 179) Reagent used to extract silver from \[A{{g}_{2}}S\] is
A)
\[NaCN\]
done
clear
B)
\[NaCN\] in presence of\[{{O}_{2}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[AgN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 180) Number of moles of \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] reduced by one mole of\[S{{n}^{2+}}\]?
A)
\[\frac{1}{3}\]
done
clear
B)
\[3\]
done
clear
C)
\[\frac{1}{6}\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 181) \[{{S}_{N}}2\]mechanism is involved in the following substitution
A)
\[C{{H}_{3}}-C{{H}_{2}}-Cl+O{{H}^{-}}\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,l-C{{H}_{3}}+O{{H}^{-}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}+O{{H}^{-}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}+O{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 182) Radioactive isotope of hydrogen is
A)
uranium
done
clear
B)
deuterium
done
clear
C)
tritium
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 183) Electronic configuration of hydride ion is
A)
\[1{{s}^{0}}\]
done
clear
B)
\[1{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}}\]
done
clear
D)
\[1{{s}^{1}}2{{s}^{1}}\]
done
clear
View Answer play_arrow
question_answer 184) Suitable conditions for melting of ice
A)
high temperature and high pressure
done
clear
B)
high temperature and low pressure
done
clear
C)
low temperature and tow pressure
done
clear
D)
low temperature and high pressure
done
clear
View Answer play_arrow
question_answer 185) Least ionized salt is
A)
\[KCl\]
done
clear
B)
\[AgCl\]
done
clear
C)
\[MgC{{l}_{2}}\]
done
clear
D)
\[CaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 186) Difference in the inching and boiling points of inert gases are
A)
large
done
clear
B)
small
done
clear
C)
no difference
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 187) Essential component of amalgam is
A)
\[Fe\]
done
clear
B)
\[Pb\]
done
clear
C)
\[Hg\]
done
clear
D)
\[Cr\]
done
clear
View Answer play_arrow
question_answer 188) Following is estimated by Liebig method
A)
\[C\]and\[H\]
done
clear
B)
nitrogen
done
clear
C)
chlorine
done
clear
D)
bromine
done
clear
View Answer play_arrow
question_answer 189) Inorganic graphite is
A)
\[BN\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[{{B}_{2}}{{H}_{6}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 190) Nausadar is
A)
\[N{{H}_{4}}N{{O}_{3}}\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\]
done
clear
D)
\[N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 191) Number of unpaired electrons in \[{{O}_{2}}\] molecule is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 192) Anode in the galvanic cell, is
A)
negative electrode
done
clear
B)
positive electrode
done
clear
C)
neutral electrode
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 193) Normal solution is
A)
inert solution
done
clear
B)
acidic solution
done
clear
C)
one litre containing one equivalent
done
clear
D)
basic solution
done
clear
View Answer play_arrow
question_answer 194) In exothermic reaction, heat is
A)
given out
done
clear
B)
absorbed
done
clear
C)
not involved
done
clear
D)
given out or absorbed
done
clear
View Answer play_arrow
question_answer 195) Element having maximum electron affinity is
A)
fluorine
done
clear
B)
chlorine
done
clear
C)
bromine
done
clear
D)
iodine
done
clear
View Answer play_arrow
question_answer 196) Bond order in benzene is
A)
1
done
clear
B)
2
done
clear
C)
1.5
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 197) Phenol and benzoic acid may be distinguished by
A)
\[NaHC{{O}_{3}}\]
done
clear
B)
\[NaOH\]
done
clear
C)
\[Na\]
done
clear
D)
\[PC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 198) Correct order of basic nature is
A)
\[N{{H}_{3}}>C{{H}_{3}}N{{H}_{2}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}>N{{H}_{3}}>C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}N{{H}_{2}}>N{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 199) Element having maximum ionization energy
A)
\[Na\]
done
clear
B)
\[Li\]
done
clear
C)
\[K\]
done
clear
D)
\[Rb\]
done
clear
View Answer play_arrow
question_answer 200) \[HCHO\]reacts with \[N{{H}_{3}}\] to give
A)
urotropine
done
clear
B)
bakelite
done
clear
C)
terylene
done
clear
D)
glyptal
done
clear
View Answer play_arrow
question_answer 201) Which of the following is a correct match?
A)
Downs syndrome - 21st chromosome
done
clear
B)
Sickel cell anaemia- X-chromosome
done
clear
C)
Haemophilia- Y-chromosome
done
clear
D)
Parkinson disease - X and Y-chromosome
done
clear
View Answer play_arrow
question_answer 202) Hydrolytic enzymes which act at low pH are called as
A)
proteases
done
clear
B)
\[\alpha \]-amylases
done
clear
C)
hydrolases
done
clear
D)
peroxidase
done
clear
View Answer play_arrow
question_answer 203) Acromegaly is caused by
A)
excess of STH
done
clear
B)
excess of thyroxine
done
clear
C)
deficiency of thyroxine
done
clear
D)
excess of adrenaline
done
clear
View Answer play_arrow
question_answer 204) Gentric drift operates in
A)
small isolated population
done
clear
B)
large isolated population
done
clear
C)
fast reproductive population
done
clear
D)
slow reproductive population
done
clear
View Answer play_arrow
question_answer 205) Mainly which type of hormones control the menstrual cycle in human beings?
A)
FSH
done
clear
B)
I.H
done
clear
C)
FSH, LH, Oestrogen
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 206) When both ovaries are removed from rat which hormone is decreased in blood
A)
oxylocin
done
clear
B)
prolactin
done
clear
C)
Ostrogen
done
clear
D)
gonadotrophic releasing factor
done
clear
View Answer play_arrow
question_answer 207) Which cartilage is present on the end of long bones ?
A)
Calcified cartilage
done
clear
B)
Hyaline cartilage
done
clear
C)
Elastic cartilage
done
clear
D)
Fibrous cartilage
done
clear
View Answer play_arrow
question_answer 208) In fluid mosaic model of plasma membrane
A)
upper layer is non-polar and hydrophilic
done
clear
B)
polar layer is hydrophobic
done
clear
C)
phospholipids form a bimolecular layer middle part
done
clear
D)
proteins from a middle layer
done
clear
View Answer play_arrow
question_answer 209) According to fossils discovered upto present time, origin and evolution of man was started from which country?
A)
France
done
clear
B)
Java
done
clear
C)
Africa
done
clear
D)
China
done
clear
View Answer play_arrow
question_answer 210) Which of the following statement is true for lymph?
A)
WBC and serum
done
clear
B)
All components of blood except RBCs and some proteins
done
clear
C)
RBCs, WBCs and Plasma
done
clear
D)
RBCs, Proteins and Platelets
done
clear
View Answer play_arrow
question_answer 211) Impulse of heart beat originates from
A)
SA node
done
clear
B)
AV node
done
clear
C)
vagus nerve
done
clear
D)
cardiac nerve
done
clear
View Answer play_arrow
question_answer 212) Continuous bleeding from an injured part of body is due to deficiency of
A)
Vitamin-A
done
clear
B)
vitamin-B
done
clear
C)
vitamin-K
done
clear
D)
vitamin-E
done
clear
View Answer play_arrow
question_answer 213) Which of the following statement is correct about the node of Ranvier?
A)
Axolemm is discontinuous
done
clear
B)
Myelin sheath is discontinuous
done
clear
C)
Both neurilemma and myelin sheath are discontinuous
done
clear
D)
Covered by myelin sheath
done
clear
View Answer play_arrow
question_answer 214) Number of wild life is continuously decreasing. What is the main reason of this?
A)
Predation
done
clear
B)
Cutting down of forests
done
clear
C)
Destruction of habitats
done
clear
D)
Hunting
done
clear
View Answer play_arrow
question_answer 215) In which era reptiles were dominated?
A)
Coenozoic era
done
clear
B)
Meszoic era
done
clear
C)
Palaeozoic era
done
clear
D)
Archazoic era
done
clear
View Answer play_arrow
question_answer 216) What is true for individual of same species?
A)
Live in same niche
done
clear
B)
Live in same habitat
done
clear
C)
Interbreeding
done
clear
D)
Live in different habitats
done
clear
View Answer play_arrow
question_answer 217) Which of the following is absent in polluted water?
A)
Hydrilla
done
clear
B)
Water hyacinth
done
clear
C)
Larva of stone fly
done
clear
D)
Blue green algae
done
clear
View Answer play_arrow
question_answer 218) In Protozoa like Amoeba and Paramecium an organelle is found for osmoregulation which is
A)
contractile vacuole
done
clear
B)
mitochondria
done
clear
C)
nucleus
done
clear
D)
food vacuole
done
clear
View Answer play_arrow
question_answer 219) During its formation, bread becomes porous due to release of \[C{{O}_{2}}\] by the action of
A)
yeast
done
clear
B)
bacteria
done
clear
C)
virus
done
clear
D)
protoxoans
done
clear
View Answer play_arrow
question_answer 220) Which bacteria are utilized in gober gas plant?
A)
Methanogens
done
clear
B)
Nitrifying bacteria
done
clear
C)
Ammonifying bacteria
done
clear
D)
Denitrifying bacteria
done
clear
View Answer play_arrow
question_answer 221) Which of the following is without exception in angiosperms?
A)
Presence of vessels
done
clear
B)
Double fertilization
done
clear
C)
Secondary growth
done
clear
D)
Autotrophic nutrition
done
clear
View Answer play_arrow
question_answer 222) Cancerous cells can easily be destroyed by radiation due to
A)
rapid cell division
done
clear
B)
lack of nutrition
done
clear
C)
fast mutation
done
clear
D)
lack of oxygen
done
clear
View Answer play_arrow
question_answer 223) Which of the following does not secrete toxins during storage conditions of crop plants?
A)
Aspergillus
done
clear
B)
Penicillium
done
clear
C)
Fusarium
done
clear
D)
Colletotrichum
done
clear
View Answer play_arrow
question_answer 224) Which of the following plant produces seeds but not flowers?
A)
Maize
done
clear
B)
Mint
done
clear
C)
Peepal
done
clear
D)
Pinus
done
clear
View Answer play_arrow
question_answer 225) Best material for the study of mitosis in laboratory is
A)
anther
done
clear
B)
root tip
done
clear
C)
leaf tip
done
clear
D)
ovary
done
clear
View Answer play_arrow
question_answer 226) Mitotic spindle is mainly composed of which proteins?
A)
Actin
done
clear
B)
Myosin
done
clear
C)
Actomyosin
done
clear
D)
Myoglobin
done
clear
View Answer play_arrow
question_answer 227) Which of the following occurs more than one and less than five in a chromosome?
A)
Chromalid
done
clear
B)
Chromomcre
done
clear
C)
Cenlromere
done
clear
D)
Telomere
done
clear
View Answer play_arrow
question_answer 228) Organisms which obtain energy by the oxidation of reduced inorganic compound are called
A)
photoautotrophs
done
clear
B)
chemoautotrophs
done
clear
C)
saprozoic
done
clear
D)
coproheterotrophs
done
clear
View Answer play_arrow
question_answer 229) In angiosperms, all the four microspores of tetrad are covered by a layer which is formed by
A)
pectocellulose
done
clear
B)
callose
done
clear
C)
cellulose
done
clear
D)
sporopollenin
done
clear
View Answer play_arrow
question_answer 230) Which type of association is found in between entomophilous flower and pollinating agent?
A)
Mutualism
done
clear
B)
Commensalism
done
clear
C)
Cooperation
done
clear
D)
Co-evolution
done
clear
View Answer play_arrow
question_answer 231) What is the direction of micropyle in anatropous ovule?
A)
Upward
done
clear
B)
Downward
done
clear
C)
Right
done
clear
D)
Left
done
clear
View Answer play_arrow
question_answer 232) Maximum greenhouse gas is released by which country ?
A)
India
done
clear
B)
France
done
clear
C)
USA
done
clear
D)
Britain
done
clear
View Answer play_arrow
question_answer 233) In angiosperms, pollen tube liberates their male gametes into the
A)
central cell
done
clear
B)
antipodal cell
done
clear
C)
egg cell
done
clear
D)
synergids
done
clear
View Answer play_arrow
question_answer 234) Which of the following absorb light energy for photosynthesis?
A)
Chlorophyll
done
clear
B)
Water molecule
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
Ku BP
done
clear
View Answer play_arrow
question_answer 235) In photosynthesis energy from light reaction to dark reaction is transferred in the form of
A)
ADP
done
clear
B)
ATP
done
clear
C)
RuDP
done
clear
D)
Chlorophyll
done
clear
View Answer play_arrow
question_answer 236) Opening and closing of stomata is due to
A)
hormonal change in guard cells
done
clear
B)
change in turgor pressure of guard cells
done
clear
C)
gaseous exchange
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 237) Which pigment absorbs the red and far red light?
A)
Cytochrome
done
clear
B)
Phytochrome
done
clear
C)
Carotenoids
done
clear
D)
Chlorophyll
done
clear
View Answer play_arrow
question_answer 238) Seed dormancy is due to the
A)
ethylene
done
clear
B)
abscisic acid
done
clear
C)
IAA
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 239) Edible part in mango is
A)
mesocarp
done
clear
B)
epicarp
done
clear
C)
endocarp
done
clear
D)
epidermis
done
clear
View Answer play_arrow
question_answer 240) What is true for cleavage?
A)
Size of embryo increases
done
clear
B)
Size of cells decreases
done
clear
C)
Size of cells increases
done
clear
D)
Size of embryo decreases
done
clear
View Answer play_arrow
question_answer 241) Geocarpic fruit is
A)
potato
done
clear
B)
peanut
done
clear
C)
onion
done
clear
D)
garlic
done
clear
View Answer play_arrow
question_answer 242) In which animal nerve cell is present but brain is absent?
A)
Sponge
done
clear
B)
Earthworm
done
clear
C)
Cockroach
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 243) In bacteria, plasmid is
A)
extrachromosomal material
done
clear
B)
main DNA
done
clear
C)
non-functional DNA
done
clear
D)
repetitive gene
done
clear
View Answer play_arrow
question_answer 244) Two different species cannot live for long duration in the same niche or habitat. This law is
A)
Aliens law
done
clear
B)
Mendels law
done
clear
C)
Causes competitive exclusion principle
done
clear
D)
Weismanns theory
done
clear
View Answer play_arrow
question_answer 245) A gene is said to be dominant if
A)
it expresses its effect only in homozygous state
done
clear
B)
it expresses only in heterozygous condition
done
clear
C)
it expresses in both homozygous and heterozygous conditions
done
clear
D)
it is never expressed in any condition
done
clear
View Answer play_arrow
question_answer 246) Pleiotropic gene is,
A)
haemophilia
done
clear
B)
thalasscmia
done
clear
C)
sickle cell anaemia
done
clear
D)
colourblindness
done
clear
View Answer play_arrow
question_answer 247) Four radial vascular bundles are found in
A)
dicot root
done
clear
B)
monocot root
done
clear
C)
dicot stem
done
clear
D)
monocot stem
done
clear
View Answer play_arrow
question_answer 248) Vessels are found in
A)
all angiosperms and some gymnosperms
done
clear
B)
most of the angiosperms and few gymnosperms
done
clear
C)
all angiosperms, all gymnosperms and some pteridophytes
done
clear
D)
all pteridophytes
done
clear
View Answer play_arrow
question_answer 249) Jacob and Monod studied lactose metabolism in E. coli and proposed operon concept. Operon concept applicable for
A)
all prokaryotes
done
clear
B)
all prokaryotes and some cukaryotes
done
clear
C)
all prokaryotic and all eukaryotic
done
clear
D)
all prokaryoics and some protozoans
done
clear
View Answer play_arrow
question_answer 250) A diseased man marries a normal woman and they get three daughters and five sons. All the daughters were diseased and sons were normal. The gene of this diseases is
A)
sex-linked dominant
done
clear
B)
sex-linked recessive
done
clear
C)
sex-limited character
done
clear
D)
autosomal dominant
done
clear
View Answer play_arrow
question_answer 251) In a DNA perecame fithymine is 20. What is the percentage of guanine?
A)
20%
done
clear
B)
40%
done
clear
C)
30%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 252) Which of the following is the example of sex-linked disease?
A)
AIDS
done
clear
B)
Colorblindness
done
clear
C)
Syphilis
done
clear
D)
Gonorrhoea
done
clear
View Answer play_arrow
question_answer 253) Which statements are correct for bacterial transduction?
A)
Transfer of some genes from one bacteria to another bacteria through virus
done
clear
B)
Transfer of genes from one bacteria to another bacteria by conjugation
done
clear
C)
Bacteria obtained its DNA directly
done
clear
D)
Bacteria obtained DNA from other external source
done
clear
View Answer play_arrow
question_answer 254) Transformation experiment was first performed on which bacteria?
A)
E. coli
done
clear
B)
Diplococcus pneumonia
done
clear
C)
Salmonella
done
clear
D)
Pasteurella pestis
done
clear
View Answer play_arrow
question_answer 255) A plant of \[{{F}_{1}}\] generation has genotype AABbCC. On selfing of this plant, the phenorypic ratio in \[{{F}_{2}}\] generation will be
A)
3 : 1
done
clear
B)
1 : 1
done
clear
C)
9 : 3 : 3 : 1
done
clear
D)
27 : 9 : 9 : 9 : 3 : 3 : 3 : 1
done
clear
View Answer play_arrow
question_answer 256) Axillary bud and terminal bud are derived from the activity of
A)
lateral meristem
done
clear
B)
intercalary meristem
done
clear
C)
apical meristem
done
clear
D)
parenchyma
done
clear
View Answer play_arrow
question_answer 257) Which of the following crops have been brought to India from New world?
A)
Cashewnut, potato, rubber
done
clear
B)
Mango, tea
done
clear
C)
Tea, rubber mango
done
clear
D)
Coffee
done
clear
View Answer play_arrow
question_answer 258) Which of the following enzymes are used to join bits of DNA ?
A)
Ligase
done
clear
B)
Primase
done
clear
C)
DNA polymerase
done
clear
D)
Endonuclease
done
clear
View Answer play_arrow
question_answer 259) Which of the following statement is true?
A)
Vessels are multicellular and with wide lumen
done
clear
B)
Tracheids are multicellular and with narrow lumen
done
clear
C)
Vessels are unicellular and with narrow lumen
done
clear
D)
Tracheids are unicellular and with wide lumen
done
clear
View Answer play_arrow
question_answer 260) Which of the following reunites the exon segments after RNA splicing?
A)
RNA polymerase
done
clear
B)
RNA primase
done
clear
C)
RNA ligase
done
clear
D)
RNA protease
done
clear
View Answer play_arrow
question_answer 261) There are three genes a, b, c, percentage of crossing over between a and b is 20%. b and c is 28% and a and c is 8%. What is the sequence of genes on chromosome?
A)
b, a, c
done
clear
B)
a, b, c
done
clear
C)
a, c, b
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 262) Manipulation of DNA in generic engineering became possible due to the discovery of
A)
restriction endonuclease
done
clear
B)
DNA ligase
done
clear
C)
transcriptase
done
clear
D)
primase
done
clear
View Answer play_arrow
question_answer 263) Convergent evolution is illustrated by
A)
starfish and cuttle fish
done
clear
B)
dogfish and whale
done
clear
C)
rat and dog
done
clear
D)
bacterium and protozoan
done
clear
View Answer play_arrow
question_answer 264) Which one of the following is a matching pair of an animal and a certain phenomenon it exhibits?
A)
Chameleon - Mimicry
done
clear
B)
Taenia - Polymorphism
done
clear
C)
Pheretima - Sexual dimorphism
done
clear
D)
Musca - Complete metamorphosis
done
clear
View Answer play_arrow
question_answer 265) Random genetic drift in a population probably result from
A)
constant low mutation rate
done
clear
B)
large population size
done
clear
C)
highly genetically variable individuals
done
clear
D)
interbreeding within this population
done
clear
View Answer play_arrow
question_answer 266) During prolonged fasting, in what sequence are the following organic compounds used up by the body ?
A)
First carbohydrates, next proteins and lastly lipids
done
clear
B)
First proteins, next lipids and lastly carbohydrate
done
clear
C)
First carbohydrate, next fats and lastly proteins
done
clear
D)
First fats, next carbohydrates and lastly proteins
done
clear
View Answer play_arrow
question_answer 267) The apical meristem of the root is present
A)
only in adventitious roots
done
clear
B)
in all the roots
done
clear
C)
only in radicals
done
clear
D)
only in tap roots
done
clear
View Answer play_arrow
question_answer 268) Diffuse porous woods are characteristic of plant growing in
A)
temperate climate
done
clear
B)
tropics
done
clear
C)
alpine region
done
clear
D)
cold winter regions
done
clear
View Answer play_arrow
question_answer 269) Which one of the following is wrong in relation to photorespiration?
A)
It is a characteristics of \[{{C}_{4}}\] plants
done
clear
B)
Ii is a characteristics of \[{{C}_{3}}\] plants
done
clear
C)
It occurs in chloroplasts
done
clear
D)
It occurs in daytime only
done
clear
View Answer play_arrow
question_answer 270) Stomata of CAM plants
A)
open during the night and close during the day
done
clear
B)
never open
done
clear
C)
are always open
done
clear
D)
open during the day and close at night
done
clear
View Answer play_arrow
question_answer 271) The major portion of the dry weight of plants comprises of
A)
carbon, nitrogen and hydrogen
done
clear
B)
carbon, hydrogen and oxygen
done
clear
C)
nitrogen, phosphorus and potassium
done
clear
D)
calcium, magnesium and sulphur
done
clear
View Answer play_arrow
question_answer 272) In Drosophila, the sex is determined by
A)
the ratio of pairs of X-chromosomes to the pairs of amosomes
done
clear
B)
whether the egg is fertilized or develops parthenogenetically
done
clear
C)
the ratio of number of X-chromosomes to the set of autosomes
done
clear
D)
X and Y-chromosomes
done
clear
View Answer play_arrow
question_answer 273) Christmas disease is another name for
A)
Downs syndrome
done
clear
B)
sleeping sickness
done
clear
C)
haemophilia-B
done
clear
D)
hepatits-B
done
clear
View Answer play_arrow
question_answer 274) When a cluster of genes show linkage behavior they
A)
do not show independent assortment
done
clear
B)
induce cell division
done
clear
C)
do not show a chromosome map
done
clear
D)
show recombination during meiosis
done
clear
View Answer play_arrow
question_answer 275) Degeneration of a genetic code is attributed to the
A)
entire codon
done
clear
B)
third member of a codon
done
clear
C)
first member of a codon
done
clear
D)
second member of a codon
done
clear
View Answer play_arrow
question_answer 276) In a flowering plant, archesporium gives rise to
A)
wall and the tapetum
done
clear
B)
only tapetum and sporogenous cells
done
clear
C)
only the wall of the sporangium
done
clear
D)
both wall and the sporogenous cells
done
clear
View Answer play_arrow
question_answer 277) Which one of the following mineral elements plays an important role in biological nitrogen fixation?
A)
Zinc
done
clear
B)
Molybdenum
done
clear
C)
Copper
done
clear
D)
Manganese
done
clear
View Answer play_arrow
question_answer 278) In sugarcane plant \[^{14}C{{O}_{2}}\] is fixed in a malic acid, in which the enzyme that fixes \[C{{O}_{2}}\] is
A)
ribulose phosphate kinase
done
clear
B)
fructose phosphatase
done
clear
C)
ribulose biphosphate carboxylase
done
clear
D)
phosphoenol pyruvic acid carboxylase
done
clear
View Answer play_arrow
question_answer 279) Which one of the following is categorized under living fossils?
A)
Selaginella
done
clear
B)
Metasequoia
done
clear
C)
Pmus
done
clear
D)
Cyccu
done
clear
View Answer play_arrow
question_answer 280) Boron in green plants assists in
A)
photosynthesis
done
clear
B)
sugar transport
done
clear
C)
activation of enzymes
done
clear
D)
acting as enzyme cofactor
done
clear
View Answer play_arrow
question_answer 281) Chlorenchyma is known to develop in the
A)
spore capsule of a moss
done
clear
B)
pollen tube of Pinus
done
clear
C)
cytoplasm of Chlorella
done
clear
D)
mycelium of a green mould such as Aspergillus
done
clear
View Answer play_arrow
question_answer 282) The aleurone layer in maize grain is specially rich in
A)
lipids
done
clear
B)
auxins
done
clear
C)
proteins
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 283) Given below are four matchings of an animal and its kind of respiratory organ (A) silver fish - trachea (B) scorpion - book lung (C) sea squirt - pharyngcal gills (D) dolphin - skin The correct matchings are
A)
B and D
done
clear
B)
C and D
done
clear
C)
A and D
done
clear
D)
A, B and C
done
clear
View Answer play_arrow
question_answer 284) Which of the following discoveries resulted in a Nobel Prize?
A)
Recombination of linked genes
done
clear
B)
Genetic engineering
done
clear
C)
X-rays induce sex-linked recessive lethal mutations
done
clear
D)
Cytoplasmic inheritance
done
clear
View Answer play_arrow
question_answer 285) In which kingdom would you classify the Archaea and nitrogen-fixing organisms. If the five-kingdom system of classification is used
A)
Protista
done
clear
B)
Monera
done
clear
C)
Plantae
done
clear
D)
Fungi
done
clear
View Answer play_arrow
question_answer 286) Which one of the following traits of garden pea studied by Mendel was a recessive feature ?
A)
Green pod colour
done
clear
B)
Round seed shape
done
clear
C)
Axial flower position
done
clear
D)
Green seed colour
done
clear
View Answer play_arrow
question_answer 287) Juicy hair-like structures observed in the lemon fruit develop from
A)
endocarp
done
clear
B)
both (a) and (b)
done
clear
C)
exocarp
done
clear
D)
mesocarp
done
clear
View Answer play_arrow
question_answer 288) Which one of the following describes correctly the homologous structures ?
A)
Organs that have no function now, but had an important function in ancestors
done
clear
B)
Organs appearing only in embryonic stage and disappearing later in the adult
done
clear
C)
Organs with anatomical similarities, but performing different functions
done
clear
D)
Organs with anatomical dissimilarities, but performing same functions
done
clear
View Answer play_arrow
question_answer 289) Darwin in his Natural Selection Theory, did not believe in any role of which one of the following in organic evolution?
A)
Struggle for existence
done
clear
B)
Discontinuous variations
done
clear
C)
Parasites and predators as natural enemies
done
clear
D)
Survival of the fittest
done
clear
View Answer play_arrow
question_answer 290) Escherichia coli is used as an indicator organism to determine pollution of water with
A)
industrial effluents
done
clear
B)
pollen of aquatic plants
done
clear
C)
heavy metals
done
clear
D)
faecal matter
done
clear
View Answer play_arrow
question_answer 291) Which one of the following pairs is not correctly matched?
A)
Vitamin \[{{B}_{12}}\] - Pernicious anaemia
done
clear
B)
Vitamin \[{{B}_{1}}\] - Beri-beri
done
clear
C)
Vitamin C - Scurvy
done
clear
D)
Vitamin \[{{B}_{2}}\] - Pellagra
done
clear
View Answer play_arrow
question_answer 292) Which elements are located at the centre of the porphyrin ring in chlorophyll?
A)
Potassium
done
clear
B)
Manganese
done
clear
C)
Calcium
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 293) In the genetic code dictionary, how many codons are used to code for all the 20 essential amino acids?
A)
61
done
clear
B)
60
done
clear
C)
20
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 294) Systemic heart refers to
A)
entire heart in lower vertebrates
done
clear
B)
the two ventricles together in humans
done
clear
C)
the heart that contracts under stimulation from nervous system
done
clear
D)
left auricle and left ventricle in higher vertebrates
done
clear
View Answer play_arrow
question_answer 295) In which one of the following options the two names refer to one and the same thing?
A)
Citric acid cycle and Calvin cycle
done
clear
B)
Tricarboxylic acid cycle and urea cycle
done
clear
C)
Krebs cycle and Calvin cycle
done
clear
D)
Tricarboxylic acid cycle and citric acid cycle
done
clear
View Answer play_arrow
question_answer 296) Test tube baby means a baby born when
A)
the ovum is fertilized externally and thereafter implanted in the uterus
done
clear
B)
it developes from a non-fertilized egg
done
clear
C)
it is developed in a test tube
done
clear
D)
it is developed through tissue culture method
done
clear
View Answer play_arrow
question_answer 297) During embryonic development, the establishment of polarity along anterior/posterior, dorsal/ventral or medial/lateral axis is called
A)
anamorphosis
done
clear
B)
pattern formation
done
clear
C)
organizer phenomena
done
clear
D)
axis formation
done
clear
View Answer play_arrow
question_answer 298) Two crosses between the same pair of genotypes or phenotypes in which the sources of the gametes are reversed in one cross, is known as
A)
dihybrid cross
done
clear
B)
reverse cross
done
clear
C)
test cross
done
clear
D)
reciprocal cross
done
clear
View Answer play_arrow
question_answer 299) Pattern baldness, moustaches and beard in human males arc examples of
A)
sex differentiating traits
done
clear
B)
sex determining traits
done
clear
C)
sex linked trails
done
clear
D)
sex limited traits
done
clear
View Answer play_arrow
question_answer 300) Plants reproducing by spores such as mosses and ferns are grouped under the general term
A)
sporophytes
done
clear
B)
thatlophytes
done
clear
C)
cryptogams
done
clear
D)
bryophyles
done
clear
View Answer play_arrow