question_answer 1) An electron is moving in magnetic field of \[16\,wb/{{m}^{2}}\]. Find the radium of its path:
A)
\[4.47\times {{10}^{11}}\,per\,\sec \]
done
clear
B)
\[{{10}^{11}}\,per\,\sec \]
done
clear
C)
\[5.3\times {{10}^{-11}}\,per\,\sec \]
done
clear
D)
\[{{10}^{14}}\,per\,\sec \]
done
clear
View Answer play_arrow
question_answer 2) Which of the following is proportional to energy density m magnetic field B:
A)
\[\frac{1}{B}\]
done
clear
B)
\[\frac{1}{{{B}^{2}}}\]
done
clear
C)
\[B\]
done
clear
D)
\[{{B}^{2}}\]
done
clear
View Answer play_arrow
question_answer 3) Which one of the following is ferro magnetic?
A)
Co
done
clear
B)
Zn
done
clear
C)
Hg
done
clear
D)
Pt
done
clear
View Answer play_arrow
question_answer 4) Sometimes positive charged particle comes from space towards earth with high velocity. Its deviation due to the magnetic field of earth will be:
A)
towards north
done
clear
B)
towards south
done
clear
C)
towards west
done
clear
D)
towards east
done
clear
View Answer play_arrow
question_answer 5) For paramagnetic materials magnetic susceptibility is related with temperature as:
A)
\[\propto {{T}^{2}}\]
done
clear
B)
\[\propto {{T}^{1}}\]
done
clear
C)
\[\propto {{T}^{-1}}\]
done
clear
D)
\[\propto {{T}^{2}}\]
done
clear
View Answer play_arrow
question_answer 6) Dynamo which produces electricity, is a application of:
A)
gravity
done
clear
B)
magnetism
done
clear
C)
e.m.f.
done
clear
D)
electrolysis
done
clear
View Answer play_arrow
question_answer 7) Air cored choke coil and delectric bulb are connected in series with A.C. mains. A soft iron rod is inserted in coil then the intensity of electric bulb will be :
A)
decrease
done
clear
B)
increase
done
clear
C)
fluctuate
done
clear
D)
unchanged
done
clear
View Answer play_arrow
question_answer 8) The orbital speed of a satellite revolving nearby the earth is:
A)
\[\sqrt{2gR}\]
done
clear
B)
\[\sqrt{gR}\]
done
clear
C)
\[\sqrt{g/R}\]
done
clear
D)
\[\sqrt{2g/R}\]
done
clear
View Answer play_arrow
question_answer 9) Which one of the following is unstable?
A)
\[\text{-}\]ray
done
clear
B)
\[\text{-}\]ray
done
clear
C)
Proton
done
clear
D)
Neutron
done
clear
View Answer play_arrow
question_answer 10) A torque of 30 N-m is acting on a wheel of mass 5 kg and moment of inertia 2 kg-m2. If wheel starts rotating from rest then, its angular displacement in 10 seconds will be:
A)
750 rad
done
clear
B)
1500 rad
done
clear
C)
3000 rad
done
clear
D)
6000 rad
done
clear
View Answer play_arrow
question_answer 11) A second pendulum is kept in a satellite it is revolving around the earth at the height of 3R from the earth surface. Time period of second pendulum will be:
A)
zero
done
clear
B)
\[3\sqrt{3\sec }\]
done
clear
C)
4 sec
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 12) A disc is rolling on an inclined plane- What fraction of its total energy will be as rotational energy :
A)
4/3
done
clear
B)
1/3
done
clear
C)
1/2
done
clear
D)
2/3
done
clear
View Answer play_arrow
question_answer 13) If temperature of the gas is increased to three times, then its root mean square velocity become :
A)
3 times
done
clear
B)
9 times
done
clear
C)
\[\frac{1}{2}\]times
done
clear
D)
\[\sqrt{3}\] times
done
clear
View Answer play_arrow
question_answer 14) Surface tension of liquid is 5N/m. If a thin film of area 0.02 m2 is made in a ring then the surface energy will be :
A)
\[\text{5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}\,}}\text{J}\]
done
clear
B)
\[\text{2}\text{.5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}\,}}\text{J}\]
done
clear
C)
\[\text{1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-1}\,}}\text{J}\]
done
clear
D)
\[\text{5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-1}\,}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 15) Which has highest penetrating power?
A)
\[\text{-}\]rays
done
clear
B)
\[\text{-}\]rays
done
clear
C)
\[\alpha \text{-}\]rays
done
clear
D)
Cathode rays
done
clear
View Answer play_arrow
question_answer 16) If X-rays is passed through from strong magnetic field, then X-rays:
A)
will deviate maximum
done
clear
B)
will deviate minimum
done
clear
C)
undeviated
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 17) In diffraction radius of half period zone is proportional to :
A)
\[{{n}^{-1/2}}\]
done
clear
B)
\[{{n}^{1/2}}\]
done
clear
C)
\[{{n}^{2}}\]
done
clear
D)
\[n\]
done
clear
View Answer play_arrow
question_answer 18) Surface tension of a liquid is T- If its surface area is increased by A, then the increase in surface energy will be :
A)
\[AT\]
done
clear
B)
\[A/T\]
done
clear
C)
\[{{A}^{2}}T\]
done
clear
D)
\[{{A}^{2}}{{T}^{2}}\]
done
clear
View Answer play_arrow
question_answer 19) If the radius of earth is decreased by 1% and mass remain constant, then the acceleration due to gravity:
A)
decrease by 2%
done
clear
B)
decrease by 1%
done
clear
C)
increase by 1%
done
clear
D)
increase by 2%
done
clear
View Answer play_arrow
question_answer 20) A sound wave of frequency 660 Hz is incident normally at reflecting wall then minimum distance from wall at which particles amplitude will be maximum (velocity of sound = 330 m/s):
A)
0.215 m
done
clear
B)
0.125 m
done
clear
C)
1 m
done
clear
D)
0.5 m
done
clear
View Answer play_arrow
question_answer 21) Different atoms of same element which have different masses but have same chemical properties are called :
A)
isochoric
done
clear
B)
isotope
done
clear
C)
isobar
done
clear
D)
isobaric
done
clear
View Answer play_arrow
question_answer 22) 22. Activity of a radioactive element is 103dis /sec. Its life is 1 sec. After 3 sec. its activity will be:
A)
1000 dis/sec
done
clear
B)
250 dis/sec
done
clear
C)
125 dis/sec
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 23) If a temperature of hot body is increased by 10%, then the increase in emitted radiation will be:
A)
10%
done
clear
B)
40%
done
clear
C)
46%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 24) Total angular momentum of a system of particles:
A)
is always constant
done
clear
B)
is changed due to presence of external force
done
clear
C)
is changed due to presence of external torque
done
clear
D)
nothing can be said
done
clear
View Answer play_arrow
question_answer 25) Ripple factor of a half wave rectifier is:
A)
1.21
done
clear
B)
0.08
done
clear
C)
0.61
done
clear
D)
2.14
done
clear
View Answer play_arrow
question_answer 26) Diffraction of sound waves is more evident than light waves in daily life because:
A)
\[{{}_{\text{sound}}}\,\text{}\,{{}_{\text{light}}}\]
done
clear
B)
\[{{}_{\text{sound}}}\,=\,{{}_{\text{light}}}\]
done
clear
C)
\[{{}_{\text{sound}}}\,<\,{{}_{\text{light}}}\]
done
clear
D)
sound waves are longitudinal but light waves are transverse
done
clear
View Answer play_arrow
question_answer 27) Two waves of intensities ratio are 9 : 1 then the ratio of their resultants maximum and minimum intensities will be:
A)
10 : 8
done
clear
B)
7 : 2
done
clear
C)
4 : 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 28) A particle is executing simple harmonic motion with angular frequency\[\omega \]then angular frequency of its kinetic energy will be:
A)
\[\omega \]
done
clear
B)
\[\omega /2\]
done
clear
C)
\[2\omega \]
done
clear
D)
\[4\omega \]
done
clear
View Answer play_arrow
question_answer 29) In a diode before the saturated condition voltage were 400 and 200 respectively. Then the respective current ratio is:
A)
\[2\sqrt{2}\]
done
clear
B)
\[\frac{1}{2\sqrt{2}}\]
done
clear
C)
\[2\]
done
clear
D)
\[1/2\]
done
clear
View Answer play_arrow
question_answer 30) 26 tuning fork are arranged in a line having beat frequency of 4 between two successive tuning forks. If frequency of last tuning fork is 3 times that of 1st tuning fork then find the frequency of 1st tuning fork:
A)
7.5 Hz
done
clear
B)
50 Hz
done
clear
C)
100 Hz
done
clear
D)
125 Hz
done
clear
View Answer play_arrow
question_answer 31) What is the value of\[\frac{R}{{{C}_{P}}}\]for a diatomic gas?
A)
5/7
done
clear
B)
2/7
done
clear
C)
3/5
done
clear
D)
3/4
done
clear
View Answer play_arrow
question_answer 32) The escape velocity of a particle of mass m varies as:
A)
\[{{m}^{2}}\]
done
clear
B)
m
done
clear
C)
\[{{m}^{0}}\]
done
clear
D)
\[{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 33) A bottle is filled with water at \[30{}^\circ C\]. When it is taken on the moon then :
A)
water will freeze
done
clear
B)
water will boil
done
clear
C)
water will decompose in hydrogen and oxygen
done
clear
D)
nothing will happen to water
done
clear
View Answer play_arrow
question_answer 34) Choose incorrect statement regards zone plate and convex lens:
A)
focal length of both depends upon wavelength\[\lambda \]
done
clear
B)
both show chromatic aberration
done
clear
C)
both have single focal length
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 35) In a potentiometer experiment the galvanometer shows no deflection when a cell is connected across 60 cm of potentiometer wire. If the cell is shunted by a resistance of 6\[\Omega \] the balance is obtained at 50 cm. Find the internal resistance of cell.
A)
0.552\[\Omega ,\]
done
clear
B)
0.652\[\Omega ,\]
done
clear
C)
1.252\[\Omega ,\]
done
clear
D)
1.2\[\Omega ,\]
done
clear
View Answer play_arrow
question_answer 36) Due to effect of interference, floating oil layer in water is visible in coloured, for observation of this event the thickness of oil layer should be:
A)
10 nm
done
clear
B)
1000 nm
done
clear
C)
1 mm
done
clear
D)
10 mm
done
clear
View Answer play_arrow
question_answer 37) A tyre at \[27{}^\circ C\] temperature and at 2 atomspheric pressure. It brusts suddenly, then the resultant temperature will be:
A)
\[-123{}^\circ C\]
done
clear
B)
\[240{}^\circ C\]
done
clear
C)
\[27{}^\circ C\]
done
clear
D)
\[0{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 38) A body takes 10 minutes to cool down from \[62{}^\circ C\] to \[50{}^\circ C\]. If the temperature of surrounding is \[26{}^\circ C\] then in the next 10 minutes temperature of the body will be:
A)
\[38{}^\circ C\]
done
clear
B)
\[40{}^\circ C\]
done
clear
C)
\[42{}^\circ C\]
done
clear
D)
\[44{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 39) During an adiabatic expansion of 2 mole of gas, the change in internal energy was, found - 50 J. The work done during the process:
A)
50 J
done
clear
B)
-50 J
done
clear
C)
100 J
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 40) Which of the following is weakest force?
A)
Gravitational
done
clear
B)
Electric force
done
clear
C)
Magnetic force
done
clear
D)
Nuclear force
done
clear
View Answer play_arrow
question_answer 41) A square is made of four rods of same material one of the diagonal of a square is at temperature difference \[100{}^\circ C\], then the temperature difference of second diagonal:
A)
\[0{}^\circ C\]
done
clear
B)
\[\frac{100}{l}\]
done
clear
C)
\[\frac{100}{2l}\]
done
clear
D)
\[100{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 42) 0.7 H inductance is connected with 220\[\Omega \]resistance with A.C. source it is connected to 50 Hz frequency and 220 volt e.m.f. source, then the value of current in circuit will be:
A)
0.5 A
done
clear
B)
0.7A
done
clear
C)
5 A
done
clear
D)
7A
done
clear
View Answer play_arrow
question_answer 43) How many times, the potential of big drop in comparison to small drops which is made of 8 droplets will be, if all the droplets are identical?
A)
2 times
done
clear
B)
4 times
done
clear
C)
3 times
done
clear
D)
8 times
done
clear
View Answer play_arrow
question_answer 44) Choke coil:
A)
decreases current in A.C.
done
clear
B)
increases current in A.C.
done
clear
C)
decreases current in D.C.
done
clear
D)
increases current in D.C.
done
clear
View Answer play_arrow
question_answer 45) Escape velocity for a rocket is 11.2 km/s. If it is taken to a planet where the radius and acceleration due to gravity is double than earth, then escape velocity will be:
A)
5.6m/s
done
clear
B)
11.2m/s
done
clear
C)
22.4 km/s
done
clear
D)
44.2 m/s
done
clear
View Answer play_arrow
question_answer 46) Potential inside a hollow sphere is:
A)
constant
done
clear
B)
proportional to distance from centre
done
clear
C)
inversely proportional to the distance
done
clear
D)
inversely proportional to square of distance
done
clear
View Answer play_arrow
question_answer 47) In p-n junction depletion region decreases when:
A)
zero bias
done
clear
B)
forward bias
done
clear
C)
reverse bias
done
clear
D)
temperature decreases
done
clear
View Answer play_arrow
question_answer 48) Hydrogen atom is excited by means of a monochromatic radiations of wavelength \[975\overset{\text{o}}{\mathop{\text{A}}}\,\]. In emission spectrum the number of possible lines are :
A)
2
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 49) When a capillary tube is immersed in water, then it rises up to height of 3 cm. If the surface tension of water is \[75\times {{10}^{-3}}N/m\]. Then the diameter of capillary tube will be:
A)
0 1 mm
done
clear
B)
0.5 mm
done
clear
C)
1 mm
done
clear
D)
2 mm
done
clear
View Answer play_arrow
question_answer 50) When two co-axial coils having same current in same direction are bring to each other, then the value of current in both coils:
A)
increases
done
clear
B)
decreases
done
clear
C)
first increases and then decreases
done
clear
D)
remain same
done
clear
View Answer play_arrow
question_answer 51) Amplification factor of triode is 18 and internal resistance is 8000\[\Omega \] If load resistance is 10, 000 \[\Omega ,\]then the voltage amplification of triode is:
A)
20
done
clear
B)
10
done
clear
C)
40
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 52) The insulation property of air breaks down at intensity as \[3\times {{10}^{6}}\,V/m\]. The maximum charge that can be given to a sphere of diameter 5 m is:
A)
\[2\times {{10}^{-2}}C\]
done
clear
B)
\[2\times {{10}^{-3}}C\]
done
clear
C)
\[2\times {{10}^{-4}}C\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 53) An observer is approaching with velocity \[\upsilon \]towards a light source- If the velocity of light is c, then velocity of light with respect to observer will be:
A)
\[c-\upsilon \]
done
clear
B)
\[c\]
done
clear
C)
\[c+\upsilon \]
done
clear
D)
\[\sqrt{1-{{\upsilon }^{2}}/{{c}^{2}}}\]
done
clear
View Answer play_arrow
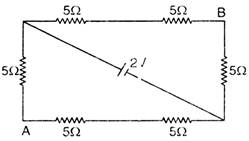
question_answer 54)
In a showing figure find the equivalent resistance between A and B.
A)
2/3 ohm
done
clear
B)
5/ ohm
done
clear
C)
8/9 ohm
done
clear
D)
7.5 ohm
done
clear
View Answer play_arrow
question_answer 55) In a circuit 20\[\Omega \] resistance and 0.4 H inductance arc connected with a source of 220 volt of frequency 50 Hz, then the value \[\phi \]is:
A)
\[{{\tan }^{-1}}(4\pi )\]
done
clear
B)
\[{{\tan }^{-1}}(2\pi )\]
done
clear
C)
\[{{\tan }^{-1}}(1\pi )\]
done
clear
D)
\[{{\tan }^{-1}}(3\pi )\]
done
clear
View Answer play_arrow
question_answer 56) Choose correct statement regarding electric lines of force:
A)
emerges from\[(-\upsilon e)\] charge and meet from \[(-\upsilon e)\]charge
done
clear
B)
where the electric lines of force are close electric field in that region is strong
done
clear
C)
Just as it is shown for a point system in the same way it represent for a solid sphere
done
clear
D)
has a physical nature
done
clear
View Answer play_arrow
question_answer 57) V= 100 sin 100t, \[I=100\] sin \[(100t+\pi /6\] then find the watt less power:
A)
\[{{10}^{4}}\,W\]
done
clear
B)
\[{{10}^{3}}\,W\]
done
clear
C)
\[{{10}^{2}}\,W\]
done
clear
D)
\[4330\,W\]
done
clear
View Answer play_arrow
question_answer 58) A galvanometer is connected with potentiometer gives null deflection, in which place current will be zero:
A)
potentiometer wire
done
clear
B)
main circuit
done
clear
C)
galvanometer circuit
done
clear
D)
battery
done
clear
View Answer play_arrow
question_answer 59) In periodic table, if elements n > 5 does not exist then total number of elements will be:
A)
50
done
clear
B)
60
done
clear
C)
110
done
clear
D)
182
done
clear
View Answer play_arrow
question_answer 60) Lenzs law represent:
A)
relation between \[I\] and B
done
clear
B)
relation between magnetic force and magnetic field
done
clear
C)
relation between e.m.f. and rate of change of flux
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 61) A cylinder of length Land radius R, whose axis is parallel to electric field. Find the total electric flux ejected from the surface of cylinder:
A)
\[2\pi {{R}^{2}}E\]
done
clear
B)
\[\pi {{R}^{2}}L/E\]
done
clear
C)
\[\pi {{R}^{2}}LE\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 62) A person is observing of two trains each of velocity is 4 m/s. A train is coming towards an observer frequency of each whistle is 240 Hz then the beats heard by an observer will be : (\[\upsilon \] = 320 m/s)
A)
zero
done
clear
B)
3
done
clear
C)
6
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 63) Diffraction pattern for a plane progressive wave is obtained from the edges of a disc. If screen is taken nearby the disc then the intensity of diffraction pattern will be:
A)
increases
done
clear
B)
decreases
done
clear
C)
remain unchanged
done
clear
D)
first increases and then decreases
done
clear
View Answer play_arrow
question_answer 64) Two ions\[A:{{Z}_{1}}=24\] and charge = e and B \[:{{Z}_{2}}=22\] and charge = 2e are enter in a transverse uniform magnetic field with same velocity in mass spectrograph then the ratio of their radii are:
A)
\[\frac{11}{24}\]
done
clear
B)
\[\frac{12}{11}\]
done
clear
C)
\[\frac{11}{22}\]
done
clear
D)
\[\frac{24}{11}\]
done
clear
View Answer play_arrow
question_answer 65) If prism angle\[\alpha =1{}^\circ ,\,\mu =1.54,\] distance between screen and prism \[(b)=0.7\,m,\] distance between prism and source \[a=0.3\,m,\] \[\lambda =180\pi \] nm then in Fresnal \[\] biprism find the value of P (finge width):
A)
\[{{10}^{-4}}m\]
done
clear
B)
\[{{10}^{-3}}mm\]
done
clear
C)
10-4\[\times \pi m\]
done
clear
D)
\[\pi \times \]10-3m
done
clear
View Answer play_arrow
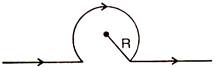
question_answer 66)
Magnetic field at point 0 will be :
A)
\[\frac{{{\mu }_{0}}I}{2R}\]interior
done
clear
B)
\[\frac{{{\mu }_{0}}I}{2R}\]exterior
done
clear
C)
\[\frac{{{\mu }_{0}}I}{2R}\left( 1-\frac{1}{\pi } \right)\]
done
clear
D)
\[\frac{{{\mu }_{0}}I}{2R}\left( 1+\frac{1}{\pi } \right)\]exterior
done
clear
View Answer play_arrow
question_answer 67) Find the ratio of electric field at the position of end on and equator of a dipole:
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
1 : 1
done
clear
D)
1 : 3
done
clear
View Answer play_arrow
question_answer 68) Current\[I\]is flowing in a conducting circular loop of radius R. It is kept in a magnetic field B which is perpendicular to the plane of circular loop. Find the magnetic force acting on the loop:
A)
\[IRB\]
done
clear
B)
\[2\pi IRB\]
done
clear
C)
zero
done
clear
D)
\[\pi IRB\]
done
clear
View Answer play_arrow
question_answer 69) According to Bohrs model of hydrogen atom, relation between principal quantum number n and radius of stable orbit:
A)
\[r\propto \frac{1}{n}\]
done
clear
B)
\[r\propto n\]
done
clear
C)
\[r\propto \frac{1}{{{n}^{2}}}\]
done
clear
D)
\[r\propto {{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 70) An open organ pipe of length 33 cm, vibratos with frequency 1000 Hz. If velocity of sound in 330 m/s. Then its frequency is:
A)
fundamental frequency
done
clear
B)
first overtone of pipe
done
clear
C)
second overtone
done
clear
D)
fourth overtone
done
clear
View Answer play_arrow
question_answer 71) Find the ratio of moment inertia of a ring about an axis passing through the circumference and perpendicular to the plane to the moment inertia about its diameter:
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 72) A stone of mass 0.2 kg is tied to one end of a thread of length 0.1 m2 whirled in a vertical circle. When the stone is at the lowest point of circle, tension m thread is 52N, then velocity of the stone will be:
A)
4 m/s
done
clear
B)
5 m/s
done
clear
C)
6 m/s
done
clear
D)
7 m/s
done
clear
View Answer play_arrow
question_answer 73) The ratio of kinetic energy to total energy, for\[{{n}^{th}}\] shell of an atom will be:
A)
1
done
clear
B)
-1
done
clear
C)
\[_{l}2\]
done
clear
D)
\[\frac{1}{{{n}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 74) 40 W, 100 W and 200 W bulbs are connected with a source of 200 V. Rating of all bulbs arc also 200 V, Now, they are connected in series, then which bulbs will glow more:
A)
300W
done
clear
B)
100W
done
clear
C)
40 W
done
clear
D)
all gives same light
done
clear
View Answer play_arrow
question_answer 75) Two simple pendulum first of bob mass \[{{M}_{1}}\] and length \[{{L}_{1}}\] second of bob mass \[{{M}_{2}}\] and length \[{{L}_{2.}}\,{{M}_{1}}={{M}_{2}}\]and \[{{L}_{1}}=2{{L}_{2.}}\]If these vibrational energy of both is same. Then which is correct?
A)
Amplitude of B greater than A
done
clear
B)
Amplitude of B smaller than A
done
clear
C)
Amplitudes will be same
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 76) n equal cells having e.m.f. E and internal resistance r, are connected in circuit of a resistance R. Same current flows in circuit either they connected in series or parallel, if:
A)
\[R=nr\]
done
clear
B)
\[R=\frac{r}{n}\]
done
clear
C)
\[R={{n}^{2}}r\]
done
clear
D)
\[R=r\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following wavelength is not possible for an X-ray tube which is operated at 40 kV.
A)
\[0.25\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.5\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.52\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 78) Transistor is more useful than triode-because:
A)
transistor are very small in size
done
clear
B)
in transistor bearing capacity of heat is less than triode
done
clear
C)
there is not need of heating in transistor
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 79) A stone is attached to the end of a string and whirled in horizontal circle, then :
A)
its linear and angular momentum are constant
done
clear
B)
only linear momentum is constant
done
clear
C)
its angular momentum is constant but linear momentum is variable
done
clear
D)
both are variable
done
clear
View Answer play_arrow
question_answer 80) A particle is executing S.H.M. of frequency 300 Hz and with amplitude 0.1 cm. Its maximum velocity will be:
A)
\[60\,\pi \,cm/s\]
done
clear
B)
\[0.6\,\pi \,cm/s\]
done
clear
C)
\[0.50\,\pi \,cm/s\]
done
clear
D)
\[0.05\,\pi \,cm/s\]
done
clear
View Answer play_arrow
question_answer 81) Which type of losses occurs in transformer?
A)
Mechanical
done
clear
B)
Copper
done
clear
C)
Hysterists
done
clear
D)
Eddy current
done
clear
View Answer play_arrow
question_answer 82) A sound wave of frequency 5Hz, has velocity 360 m/s. If phase difference between two particles is\[60{}^\circ \], then path difference will be:
A)
1.2m
done
clear
B)
12m
done
clear
C)
2.1 m
done
clear
D)
0.21m
done
clear
View Answer play_arrow
question_answer 83) Positively charged rays/particles are :
A)
\[\text{-}\]rays
done
clear
B)
neutron
done
clear
C)
ion
done
clear
D)
electrons magnetic radiation
done
clear
View Answer play_arrow
question_answer 84) A body is rotating with uniform angular acceleration. If it starts from rest then after time t, angular displacement is proportional to:
A)
\[\sqrt{t}\]
done
clear
B)
\[t\]
done
clear
C)
\[{{t}^{3}}\]
done
clear
D)
\[{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 85) The time period of a simple pendulum in a stationary lift 10 T. when the lift falls freely, then its time period will be:
A)
T
done
clear
B)
2T
done
clear
C)
\[\infty \]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 86) Linear density of a string is \[1.3\times {{10}^{-4}}\,kg/m\] and wave equation is y = 0.021 sin (x + 30 0. Find the tension in the string where x in metre and t in second:
A)
0.12 N
done
clear
B)
0.21 N
done
clear
C)
1.2 N
done
clear
D)
0.012 N
done
clear
View Answer play_arrow
question_answer 87) Point of suspension and point of oscillation have:
A)
same time period and can be inter changeable
done
clear
B)
same time period and non inter changeable
done
clear
C)
unequal time period and can be inter changeable
done
clear
D)
unequal time period and in inter changeable
done
clear
View Answer play_arrow
question_answer 88) Graph between absolute temperature T and square of sound velocity will be:
A)
circular
done
clear
B)
elliptical
done
clear
C)
straight line with positive slope
done
clear
D)
straight line with negative slope
done
clear
View Answer play_arrow
question_answer 89)
If diode is ideal then potential at A will be:
A)
0.0V
done
clear
B)
0.3V
done
clear
C)
0.7V
done
clear
D)
6.0V
done
clear
View Answer play_arrow
question_answer 90) If a light wave passes through transparent medium (like as glass). Then:
A)
velocity of all light waves will be same
done
clear
B)
velocity of longer wavelength will be
done
clear
C)
velocity of longest wavelength will be maximum
done
clear
D)
velocity of shorter wavelength will be maximum
done
clear
View Answer play_arrow
question_answer 91) Length of a potentiometer wire is kept more and uniform to achieve :
A)
uniform and more potential gradient
done
clear
B)
non-uniform and more potential gradient
done
clear
C)
uniform and less potential gradient
done
clear
D)
non-uniform and less potential gradient
done
clear
View Answer play_arrow
question_answer 92) When temperature of a gas is increased, then which of the following is always true:
A)
work is done on the gas
done
clear
B)
heat is supplied to gas
done
clear
C)
energy of gas is increased
done
clear
D)
pressure of a gas does not increased
done
clear
View Answer play_arrow
question_answer 93) Which of the following statement is incorrect according to assumptions of kinetic theory of gases?
A)
Potential energy of a molecule is zero
done
clear
B)
Molecules moves randomly in all directions
done
clear
C)
Kinetic energy of molecules change when they collids with wall of container
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 94) Electromagnets are made of soft iron (because soft iron have :
A)
low retentivity and high susceptibility
done
clear
B)
low retentivity and low susceptibility
done
clear
C)
high retentivity and high susceptibility
done
clear
D)
high retentivity and low susceptibility
done
clear
View Answer play_arrow
question_answer 95) The lines of magnetic induction due to a current carrying straight long conductor:
A)
parallel to wire
done
clear
B)
perpendicular to wire
done
clear
C)
concentric circular
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 96) Real power consumption in a circuit is least when it contains :
A)
high R low L
done
clear
B)
high R high L
done
clear
C)
low R high L
done
clear
D)
high R low C
done
clear
View Answer play_arrow
question_answer 97) For constant plate voltage, plate current will be maximum when :
A)
\[{{V}_{G}}+\upsilon e,{{V}_{p}}=-\upsilon e\]
done
clear
B)
\[{{V}_{G}}+\upsilon e,{{V}_{p}}=+\upsilon e\]
done
clear
C)
\[{{V}_{G}}-\upsilon e,{{V}_{p}}=+\upsilon e\]
done
clear
D)
\[{{\upsilon }_{G}}=0,\,{{V}_{p}}=+\,\upsilon e\]
done
clear
View Answer play_arrow
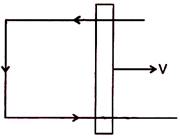
question_answer 98)
For given arrangement (in horizontal plane) the possible direction of magnetic field:
A)
towards right
done
clear
B)
towards left
done
clear
C)
vertically upward
done
clear
D)
vertically downward
done
clear
View Answer play_arrow
question_answer 99) How many unpaired electrons are present in cobalt \[[Co]\] metal?
A)
\[2\]
done
clear
B)
\[3\]
done
clear
C)
\[4\]
done
clear
D)
\[7\]
done
clear
View Answer play_arrow
question_answer 100) Isoelectronic species is:
A)
\[{{F}^{-}},{{O}^{-2}}\]
done
clear
B)
\[{{F}^{-}},O\]
done
clear
C)
\[{{F}^{-}},{{O}^{+}}\]
done
clear
D)
\[{{F}^{-}},{{O}^{+2}}\]
done
clear
View Answer play_arrow
question_answer 101) How many-ions are produce in aqueous solution of \[[Co{{({{H}_{2}}O)}_{6}}]C{{l}_{2}}\]?
A)
\[2\]
done
clear
B)
\[3\]
done
clear
C)
\[4\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 102) The magnetic quantum number of valence electron of sodium \[(Na)\] is:
A)
\[3\]
done
clear
B)
\[2\]
done
clear
C)
\[1\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 103) For the reaction, \[CaC{{O}_{3}}(s)CaO(s)+C{{O}_{2}}(g);\] \[{{K}_{P}}\]is:
A)
\[{{K}_{P}}={{P}_{(CaC{{O}_{3}})}}\]
done
clear
B)
\[{{K}_{P}}={{P}_{(C{{O}_{2}})}}\]
done
clear
C)
\[{{K}_{P}}=\frac{1}{{{P}_{(CaC{{O}_{3}})}}}\]
done
clear
D)
\[{{K}_{P}}=\frac{1}{{{P}_{(C{{O}_{2}})}}}\]
done
clear
View Answer play_arrow
question_answer 104) The relationship between ionization and change in concentration of any weak electrolyte is represented as:
A)
\[\alpha =\frac{{{K}_{a}}}{C}\]
done
clear
B)
\[\alpha =\sqrt{\frac{{{K}_{a}}}{C}}\]
done
clear
C)
\[\alpha ={{K}_{a}}.C\]
done
clear
D)
\[\alpha =\frac{\sqrt{{{K}_{a}}}}{{{C}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 105) Which compound decolorizes iodine solution?
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[N{{a}_{2}}S\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
D)
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 106) Which quantum number is not related with Schrodinger equation?
A)
Principal
done
clear
B)
Azimuthal
done
clear
C)
Magnetic
done
clear
D)
Spin
done
clear
View Answer play_arrow
question_answer 107) Which of following is not Lewis base?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 108) An element have atomic weight 40 and its electronic configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}\] . Then its atomic number and number of neutrons will be:
A)
\[18\] and \[22\]
done
clear
B)
\[22\] and \[18\]
done
clear
C)
\[20\] and \[20\]
done
clear
D)
\[40\] and \[18\]
done
clear
View Answer play_arrow
question_answer 109) An alcoholic drinks substance \[pH=4.7\] then \[^{-}OH\] ion concentration of this solution is:
A)
\[3\times {{10}^{-10}}\]
done
clear
B)
\[5\times {{10}^{-10}}\]
done
clear
C)
\[1\times {{10}^{-10}}\]
done
clear
D)
\[5\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 110) Which of the following is weakest bond?
A)
Ionic
done
clear
B)
Covalent
done
clear
C)
Metallic
done
clear
D)
van der Waals
done
clear
View Answer play_arrow
question_answer 111) \[{{[Ti{{({{H}_{2}}O)}_{6}}]}^{+3}}\] is paramagnetic in nature due to:
A)
one unpaired \[{{e}^{-}}\]
done
clear
B)
Two unpaired \[{{e}^{-}}\]
done
clear
C)
three unpaired \[{{e}^{-}}\]
done
clear
D)
no unpaired \[{{e}^{-}}\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following has lowest first ionization energy?
A)
\[H\]
done
clear
B)
\[He\]
done
clear
C)
\[Xe\]
done
clear
D)
\[Li\]
done
clear
View Answer play_arrow
question_answer 113) Chlorophyll contains:
A)
\[Fe\]
done
clear
B)
\[Na\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Zn\]
done
clear
View Answer play_arrow
question_answer 114) The bond angle of water \[{{104}^{o}}31\] due to:
A)
repulsion between \[1p\] and bond pair
done
clear
B)
\[s{{p}^{3}}\]-hybridization of O
done
clear
C)
H-bonding
done
clear
D)
higher electronegativity of O
done
clear
View Answer play_arrow
question_answer 115) Maximum oxidation state of \[Cr\] is:
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[6\]
done
clear
D)
\[7\]
done
clear
View Answer play_arrow
question_answer 116) At \[298\text{ }K,\] the solubility of \[PbC{{l}_{2}}\]is \[2\times {{10}^{-2}}\text{ }mol/litre\]then \[{{K}_{sp}}=?\]:
A)
\[1\times {{10}^{-7}}\]
done
clear
B)
\[3.2\times {{10}^{-2}}\]
done
clear
C)
\[1\times {{10}^{-5}}\]
done
clear
D)
\[3.2\times {{10}^{-5}}\]
done
clear
View Answer play_arrow
question_answer 117) For \[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\] .equilibrium constant is K then equilibrium constant for \[2{{N}_{2}}+6{{H}_{2}}4N{{H}_{3}}\]:
A)
\[\sqrt{K}\]
done
clear
B)
\[{{K}^{2}}\]
done
clear
C)
\[\frac{K}{2}\]
done
clear
D)
\[\sqrt{\frac{1}{K}}+1\]
done
clear
View Answer play_arrow
question_answer 118) Which solution used as electrolyte in aluminium metal extraction?
A)
\[A{{l}_{2}}{{O}_{3}}.{{H}_{2}}O\]
done
clear
B)
\[A{{l}_{2}}{{O}_{3}}.{{H}_{2}}O\] and \[N{{a}_{3}}Al{{F}_{6}}\](molten solution)
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 119) Which of the following most reducing agent?
A)
\[HN{{O}_{3}}\]
done
clear
B)
\[Na\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[Cr\]
done
clear
View Answer play_arrow
question_answer 120) \[C{{N}^{\bigcirc -}}\] solution used in extraction of which metal:
A)
\[Ag\]
done
clear
B)
\[Ti\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Sn\]
done
clear
View Answer play_arrow
question_answer 121) What conclusion come from Rutherford \[\alpha \]-particle scattering experiment?
A)
Nucleus is made up of proton and electron
done
clear
B)
Number of electron and proton are same
done
clear
C)
Proton is small positively charged centre
done
clear
D)
Electron spin continuously in a energy shell
done
clear
View Answer play_arrow
question_answer 122) Oxidation state of \[Fe\] in \[{{K}_{3}}[Fe{{(CN)}_{6}}]\]:
A)
\[2\]
done
clear
B)
\[3\]
done
clear
C)
\[0\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 123) Conjugate base of \[N{{H}_{3}}\]is:
A)
\[N{{H}_{4}}^{\oplus }\]
done
clear
B)
\[N{{H}_{2}}^{\oplus }\]
done
clear
C)
\[N{{H}_{2}}^{\bigcirc -}\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 124) Blood pH remain constant due to presence of:
A)
acidic solution
done
clear
B)
basic solution
done
clear
C)
blood protein
done
clear
D)
buffer effect
done
clear
View Answer play_arrow
question_answer 125) Which of the following not Lewis acid?
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[FeC{{l}_{3}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
\[P{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 126) Aqueous solution of \[AlC{{l}_{3}}\] is:
A)
acidic
done
clear
B)
basic
done
clear
C)
amphoteric
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 127) Electronic configuration of \[F{{e}^{+2}}\] is:
A)
\[[Ar]\,4{{s}^{2}}\,3{{d}^{6}}\]
done
clear
B)
\[[Ar]\,\,3{{d}^{6}}\]
done
clear
C)
\[[Ar]\,\,4{{s}^{2}}3{{d}^{4}}\]
done
clear
D)
\[[Ar]\,\,4{{s}^{2}}3{{d}^{0}}\]
done
clear
View Answer play_arrow
question_answer 128) Relation between \[{{K}_{p}}\] and \[{{K}_{c}}\] is:
A)
\[{{K}_{c}}={{K}_{p}}{{(RT)}^{\Delta n}}\]
done
clear
B)
\[{{K}_{p}}={{K}_{c}}{{(RT)}^{\Delta n}}\]
done
clear
C)
\[{{K}_{c}}={{K}_{p}}{{(RT)}^{-\Delta n}}\]
done
clear
D)
both (b) and (c)
done
clear
View Answer play_arrow
question_answer 129) Indicator which not used for titration of \[{{S}_{A}}\] and \[{{S}_{B}}\]?
A)
\[MeOH\]
done
clear
B)
\[HPh\]
done
clear
C)
Aniline blue
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 130) In this reaction, \[{{I}_{2}}+{{I}^{-}}\xrightarrow{{}}{{I}_{3}}^{\bigcirc -}\]Lewis base is:
A)
\[{{I}_{2}}\]
done
clear
B)
\[{{I}^{\bigcirc -}}\]
done
clear
C)
\[{{I}_{3}}^{\bigcirc -}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 131) \[{{H}_{2}}(g)+{{I}_{2}}(g)2HI(g)\] In this reaction when increase pressure the reaction direction is:
A)
does not change
done
clear
B)
forward
done
clear
C)
backward
done
clear
D)
decrease
done
clear
View Answer play_arrow
question_answer 132) Chemical property of \[Li\] and \[Mg\] is similar because:
A)
these belong to same group
done
clear
B)
both IP is same
done
clear
C)
shows diagonal relationship
done
clear
D)
both EA is same
done
clear
View Answer play_arrow
question_answer 133) Which property does not change in a group of periodic table?
A)
Electronegativity
done
clear
B)
Atomic size
done
clear
C)
Electron affinity
done
clear
D)
Valence \[{{e}^{-}}\]
done
clear
View Answer play_arrow
question_answer 134) Correct for \[{{N}_{2}}\] triple bond:
A)
\[3\sigma \]
done
clear
B)
\[1\pi ,\,2\sigma \]
done
clear
C)
\[2\pi ,1\sigma \]
done
clear
D)
\[3\pi \]
done
clear
View Answer play_arrow
question_answer 135) If in hybrid orbital s-orbital character increases, then bond angle:
A)
increases
done
clear
B)
decreases
done
clear
C)
zero
done
clear
D)
does not change
done
clear
View Answer play_arrow
question_answer 136) Sodium gives blue colour with \[N{{H}_{3}}\] solution this blue colour due to:
A)
ammoniated \[N{{a}^{\oplus }}\]
done
clear
B)
ammoniated \[N{{a}^{\bigcirc -}}\]
done
clear
C)
ammoniated \[{{e}^{-}}\]
done
clear
D)
\[N{{a}^{+}}/N{{a}^{-}}\] pair
done
clear
View Answer play_arrow
question_answer 137) When \[{{H}_{2}}S\] is passed through a mixture containing \[C{{u}^{+2}},\] \[N{{i}^{+2}},\] \[Z{{n}^{+2}}\]in acidic solution then ion will precipitate:
A)
\[C{{u}^{+2}},N{{i}^{+2}}\]
done
clear
B)
\[N{{i}^{+2}}\]
done
clear
C)
\[C{{u}^{+2}},Z{{n}^{+2}}\]
done
clear
D)
\[C{{u}^{+2}}\]
done
clear
View Answer play_arrow
question_answer 138) pH of human blood is \[7.4\]. Then \[{{H}^{+}}\] concentration will be:
A)
\[4\times {{10}^{-8}}\]
done
clear
B)
\[2\times {{10}^{-8}}\]
done
clear
C)
\[4\times {{10}^{-4}}\]
done
clear
D)
\[2\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 139) In the conversion \[B{{r}_{2}}\to Br{{O}_{3}}^{-}\] oxidation state of bromine changes:
A)
\[0\] to \[5\]
done
clear
B)
\[1\]to \[3\]
done
clear
C)
\[2\] to\[4\]
done
clear
D)
\[5\]to \[1\]
done
clear
View Answer play_arrow
question_answer 140) Oxidation state of oxygen in \[{{H}_{2}}{{O}_{2}}\]is:
A)
\[-1\]
done
clear
B)
\[+1\]
done
clear
C)
\[-2\]
done
clear
D)
\[+2\]
done
clear
View Answer play_arrow
question_answer 141) Which compound aqueous solution is colored?
A)
\[Zn{{(N{{O}_{3}})}_{2}}\]
done
clear
B)
\[LiN{{O}_{3}}\]
done
clear
C)
\[Co{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
\[Ba{{(N{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 142) Property of ionic compound is:
A)
high M.R and high B.R
done
clear
B)
high M,R and low B.R
done
clear
C)
low M.R and high B.R
done
clear
D)
low M.R and low B.R
done
clear
View Answer play_arrow
question_answer 143) Collins reagent is:
A)
\[Mn{{O}_{2}}/HCl\]
done
clear
B)
\[Mn{{O}_{4}}/{{C}_{5}}{{H}_{5}}N\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[C{{r}_{2}}{{O}_{3}}/2{{C}_{5}}{{H}_{5}}N\]
done
clear
View Answer play_arrow
question_answer 144) On reacting with G.R. acetone gives:
A)
\[{{1}^{o}}\] alcohol
done
clear
B)
\[{{2}^{o}}\]alcohol
done
clear
C)
\[{{3}^{o}}\] alcohol
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 145) Depletion of ozone layer caused by:
A)
freon
done
clear
B)
alkane
done
clear
C)
Gringard reagent
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 146) Which compound shows dipole moment?
A)
1, 4-di-chloro benzene
done
clear
B)
1, 2 -di-chloro benzene
done
clear
C)
trans-1, 2 di-chloro ethane
done
clear
D)
frans-2-butene
done
clear
View Answer play_arrow
question_answer 147) Product formed by reaction between primary amine \[CHC{{l}_{3}}\] and alcoholic \[KOH\]:
A)
cynide
done
clear
B)
iso cynide
done
clear
C)
nitro amine
done
clear
D)
alkane
done
clear
View Answer play_arrow
question_answer 148) IUPAC name of compound is: \[C{{H}_{3}}-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{2}}-COCl\]
A)
3-methyl pentanoyl chloride
done
clear
B)
3-methyl butanoyl chloride
done
clear
C)
1-chloro-3-methyl pentanol
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 149) Which statement is true?
A)
The bp. of diethyl ether and \[{{C}_{2}}{{H}_{5}}OH\] is equal
done
clear
B)
Diethyl ether have dipole moment
done
clear
C)
Diethyl ether high dissolve in water
done
clear
D)
Diethyl ether is Lewis acid
done
clear
View Answer play_arrow
question_answer 150) What product is formed when acetic acid react with \[{{P}_{2}}{{O}_{5}}\]?
A)
Acetyl chloride
done
clear
B)
Trichloro acetic acid
done
clear
C)
Acetic anhydride
done
clear
D)
Di-chloro acetic acid
done
clear
View Answer play_arrow
question_answer 151) What product is formed when diethyl ether on exposure to sunlight and air with a long period?
A)
Peroxide
done
clear
B)
Ethyl alcohol
done
clear
C)
Di-ethyl ketone
done
clear
D)
Ethane
done
clear
View Answer play_arrow
question_answer 152) Hybridisation of O atom in ether is:
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[sp\]
done
clear
D)
\[ds{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 153) What product is formed when 1-chlorobutane react with alcoholic \[KOH\]?
A)
\[1-Butene\]
done
clear
B)
\[2-Butene\]
done
clear
C)
\[1-Butanol\]
done
clear
D)
\[2-Butaneol\]
done
clear
View Answer play_arrow
question_answer 154) Which compound give \[C{{O}_{2}}\] with\[NaHC{{O}_{3}}\]?
A)
Phenol
done
clear
B)
Phenol + acetic acid
done
clear
C)
n-butanol
done
clear
D)
Acetic acid
done
clear
View Answer play_arrow
question_answer 155) Primary \[({{1}^{o}}),\] secondary \[({{2}^{o}}),\] tertiary \[({{3}^{o}})\]alcohol order of boiling point is:
A)
\[{{1}^{o}}>{{2}^{o}}>{{3}^{o}}\]
done
clear
B)
\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\]
done
clear
C)
\[{{2}^{o}}>{{1}^{o}}>{{3}^{o}}\]
done
clear
D)
\[{{2}^{o}}>{{3}^{o}}>{{1}^{o}}\]
done
clear
View Answer play_arrow
question_answer 156) Process by which formation of acetone take place:
A)
pyrolysis of \[C{{H}_{3}}COOH\]
done
clear
B)
oxidation of \[C{{H}_{3}}COOH\]
done
clear
C)
pyrolysis of calcium acetate
done
clear
D)
oxidation of n-propyl alcohol
done
clear
View Answer play_arrow
question_answer 157) Which of the following is not a member of homologus series?
A)
Ethene
done
clear
B)
1-butene
done
clear
C)
2-butene
done
clear
D)
2-butyne
done
clear
View Answer play_arrow
question_answer 158) Calcium carbide on hydrolysis form:
A)
ethane
done
clear
B)
ethylene
done
clear
C)
methane
done
clear
D)
acetylene
done
clear
View Answer play_arrow
question_answer 159) Which of following gives \[{{H}_{2}}S\] gas with \[Na\]?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 160) Which of following exist as zwitter ion?
A)
Ammonium acetate
done
clear
B)
Ethyl acetate
done
clear
C)
Glycine
done
clear
D)
Aniline hydrochloride
done
clear
View Answer play_arrow
question_answer 161) Compound which is used for separation of acetone and acetophenone?
A)
Sodium bisulphite
done
clear
B)
Girgnard reagent
done
clear
C)
Sodium sulphate
done
clear
D)
Ammonium chloride
done
clear
View Answer play_arrow
question_answer 162) Which compound is chiral?
A)
Butane
done
clear
B)
1-Chloro-2-methyl butane
done
clear
C)
2-Methyl butane
done
clear
D)
2-Methyl propane
done
clear
View Answer play_arrow
question_answer 163) General formula of paraffin is:
A)
\[{{C}_{n}}{{H}_{2n}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{2n-2}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+2}}\]
done
clear
D)
\[{{C}_{2n}}{{H}_{2n}}\]
done
clear
View Answer play_arrow
question_answer 164) Methyl acetate and propionic acid are:
A)
functional isomer
done
clear
B)
structural isomer
done
clear
C)
stereo isomer
done
clear
D)
geometrical isomer
done
clear
View Answer play_arrow
question_answer 165) Et. \[MgBr+C{{O}_{2}}\to \]Product is:
A)
\[{{C}_{3}}{{H}_{7}}COOH\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}COOH\]
done
clear
C)
\[{{C}_{4}}{{H}_{9}}COOH\]
done
clear
D)
\[HCOOH\]
done
clear
View Answer play_arrow
question_answer 166) \[H-C\equiv C-HC=C{{H}_{2}}\] compound have hybridisation of \[C-C\] single bond is:
A)
\[s{{p}^{3}}-s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{3}}-s{{p}^{2}}\]
done
clear
C)
\[sp-s{{p}^{2}}\]
done
clear
D)
\[sp-s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 167) \[RCON{{H}_{2}}+B{{r}_{2}}+KOH\to \]main product is:
A)
\[RCN\]
done
clear
B)
\[RCON{{H}_{2}}\]
done
clear
C)
\[R-C{{H}_{2}}-N{{H}_{2}}\]
done
clear
D)
\[RN{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 168) Total isomer of \[{{C}_{4}}{{H}_{10}}\] is:
A)
zero
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 169) Which compound increases ant knocking property of gasoline?
A)
\[{{({{C}_{2}}{{H}_{5}})}_{4}}Pb\]
done
clear
B)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}Pb\]
done
clear
C)
\[{{(C{{H}_{3}})}_{4}}Pb\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}Pb\]
done
clear
View Answer play_arrow
question_answer 170) Bayers reagent is used for detection of:
A)
amines
done
clear
B)
glucose
done
clear
C)
unsaturated bond
done
clear
D)
alcohol
done
clear
View Answer play_arrow
question_answer 171) Which compound does not give Cannizzaros is reaction?
A)
N.O.T.
done
clear
B)
Benzaldehyde
done
clear
C)
Formaldehyde
done
clear
D)
Acetaldehyde
done
clear
View Answer play_arrow
question_answer 172) A and B react with \[Na\] gives \[{{H}_{2}}\] gas and by reaction of both A and B formed ethyl acetate is formed then A and B is:
A)
\[C{{H}_{3}}COOH,{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}COOH,C{{H}_{3}}OH\]
done
clear
C)
\[{{C}_{3}}{{H}_{7}}COOH,{{C}_{3}}{{H}_{7}}OH\]
done
clear
D)
\[HCOOH,C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 173) A compound \[{{C}_{5}}{{H}_{8}}\] which give white ppt. with ammonical \[AgN{{O}_{3}}\]. A give \[{{(C{{H}_{3}})}_{2}}CHCOOH\]with hot alkaline \[KMn{{O}_{4}}\]then compound is:
A)
\[C{{H}_{3}}C{{H}_{2}}-C{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-C\equiv CH\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CH-C\equiv CH\]
done
clear
D)
\[C{{H}_{2}}=CH-C{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 174) Acidic order of tri-chloroacetic acid , tri-fluro acetic acid (A), acetic acid (C) is:
A)
\[A>B>C\]
done
clear
B)
\[B>A>C\]
done
clear
C)
\[C>A>B\]
done
clear
D)
\[B=A=C\]
done
clear
View Answer play_arrow
question_answer 175) Which of the following is Teflon?
A)
\[{{[-C{{F}_{2}}-C{{F}_{2}}-]}_{n}}\]
done
clear
B)
\[C{{F}_{2}}=C{{F}_{2}}\]
done
clear
C)
\[CF\equiv CF\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 176) Product formed by oxidation of isopropyl alcohol:
A)
propene
done
clear
B)
acetyldehyde
done
clear
C)
acetone
done
clear
D)
ethane
done
clear
View Answer play_arrow
question_answer 177) Electrophilic substitution reaction in phenol take place at:
A)
para position
done
clear
B)
meta position
done
clear
C)
ortho position
done
clear
D)
o and p position
done
clear
View Answer play_arrow
question_answer 178) Which compound gives iodoform by reaction with \[{{I}_{2}}\] and \[NaOH\]?
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[{{C}_{3}}{{H}_{7}}OH\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 179) Aryl halide is less reactive than alkyl halide towards nucleophilic substitution because:
A)
less stable carbonium ion
done
clear
B)
due to large \[C-Cl\]bond energy
done
clear
C)
inductive effect
done
clear
D)
resonance stabilization and \[s{{p}^{2-}}\]hybridisation of C attached to halide
done
clear
View Answer play_arrow
question_answer 180) IUPAC name of \[C{{H}_{2}}=CH-CH{{(C{{H}_{3}})}_{2}}:\]
A)
vinyl is propane
done
clear
B)
3-methyl-1-butene
done
clear
C)
3-methyl-2-butene
done
clear
D)
1-methyl-3-butene
done
clear
View Answer play_arrow
question_answer 181) Which of the following gives most stable carbocation by dehydration?
A)
\[{{(C{{H}_{3}})}_{2}}CH-OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}C-OH\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{3}}-OH\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-O-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 182) Common name of phosgene is:
A)
phosphonyl chloride
done
clear
B)
carbonyl chloride
done
clear
C)
carbon tetra chloride
done
clear
D)
tri-phenyl phosphene
done
clear
View Answer play_arrow
question_answer 183) Glucose \[\to \] ethyl alcohol in this reaction enzyme is
A)
zymase
done
clear
B)
invertase
done
clear
C)
maltase
done
clear
D)
diastage
done
clear
View Answer play_arrow
question_answer 184) Increasing order of acidity:
A)
m-nitro phenol < p - nitro phenol < phenol < p-methyl phenol
done
clear
B)
p-methyl phenol < phenol < m-nitro phenol < nitro phenol
done
clear
C)
phenol < p-methyl phenol < p-nitro phenol < m-nitro phenol
done
clear
D)
all have same acidity
done
clear
View Answer play_arrow
question_answer 185) Nylon 66 is:
A)
polyamide
done
clear
B)
polyester
done
clear
C)
polystyrene
done
clear
D)
polyvinyl
done
clear
View Answer play_arrow
question_answer 186) Normal butane convert into isobutane by:
A)
\[LiAl{{H}_{4}}\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[NaB{{H}_{4}}\]
done
clear
D)
\[Zn/HCl\]
done
clear
View Answer play_arrow
question_answer 187) In the reaction, \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}+HCl+NaN{{O}_{2}}\xrightarrow{{}}X\]product X is:
A)
aniline hydrochloride > with the
done
clear
B)
nitro aniline
done
clear
C)
benzene diazonium chloral
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 188) What is the main product of the reaction between 2-methyl propene with \[HBr\]?
A)
1-bromo butane
done
clear
B)
1-bromo-2-methyl propane
done
clear
C)
2-bromo butane
done
clear
D)
2-bromo-2-methyl propane
done
clear
View Answer play_arrow
question_answer 189) When aliphatic aldehyde heat with Fehling solution product formed is:
A)
\[CuO\]
done
clear
B)
\[C{{u}_{2}}O\]
done
clear
C)
\[CuS{{O}_{4}}\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 190) Mesitylene is formed by:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 191) The correct identification of unsaturated double bond in alkene is done by:
A)
hydrogenation
done
clear
B)
dehydration
done
clear
C)
ozonolysis
done
clear
D)
photolysis
done
clear
View Answer play_arrow
question_answer 192) How many TC-bonds are present in napthalene molecule?
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[5\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 193) Hybridisation state of C in diamond is:
A)
\[sp\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
question_answer 194) \[2Mn{{O}_{4}}^{\bigcirc -}+5{{H}_{2}}{{O}_{2}}+6{{H}^{+}}\to 2Z+5{{O}_{2}}+8{{H}_{2}}O\] In this reaction Z is:
A)
\[M{{n}^{+2}}\]
done
clear
B)
\[M{{n}^{+4}}\]
done
clear
C)
\[Mn{{O}_{2}}^{\bigcirc -}\]
done
clear
D)
\[Mn\]
done
clear
View Answer play_arrow
question_answer 195) Which of the following element does not occur in liquid form?
A)
\[Hg\]
done
clear
B)
\[Li\]
done
clear
C)
\[Ga\]
done
clear
D)
\[Br\]
done
clear
View Answer play_arrow
question_answer 196) Co-ordinate bond is absent in:
A)
\[B{{H}_{4}}^{\bigcirc -}\]
done
clear
B)
\[C{{O}_{3}}^{-2}\]
done
clear
C)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
D)
\[N{{H}_{4}}^{\oplus }\]
done
clear
View Answer play_arrow
question_answer 197) Phenol is used in manufacture of:
A)
nylon
done
clear
B)
polystyrene
done
clear
C)
bakelite
done
clear
D)
PVC
done
clear
View Answer play_arrow
question_answer 198) Correct order of base strength is:
A)
\[E{{t}_{2}}NH>EtN{{H}_{2}}>E{{t}_{3}}N\]
done
clear
B)
\[EtN{{H}_{2}}>E{{t}_{2}}NH>E{{t}_{3}}N\]
done
clear
C)
\[E{{t}_{2}}NH>E{{t}_{3}}N>EtN{{H}_{2}}\]
done
clear
D)
all have same base strength
done
clear
View Answer play_arrow
question_answer 199) Reserpine is obtained from:
A)
Asafoetida
done
clear
B)
Rauwolfia serpentine
done
clear
C)
Curcuma longa
done
clear
D)
Pnpaver sonimferum
done
clear
View Answer play_arrow
question_answer 200) The coiling of tendril around some base in response to touch is called:
A)
hydrotaxis
done
clear
B)
chcmotaxis
done
clear
C)
thigmtropism
done
clear
D)
geotaxis
done
clear
View Answer play_arrow
question_answer 201) Sperm enters from which part of egg:
A)
anywhere in unfertilized egg from animal pole
done
clear
B)
from animal pole in unfertilized egg
done
clear
C)
in unfertilized egg from vegetal pole
done
clear
D)
none of these above
done
clear
View Answer play_arrow
question_answer 202) Seed coat is derived from:
A)
pericarp
done
clear
B)
epicarp
done
clear
C)
integuments of ovule
done
clear
D)
nucellus
done
clear
View Answer play_arrow
question_answer 203) 6 molecules glucose +6 molecules of \[{{O}_{2}}\] and 8 ADP combined to form \[6{{H}_{2}}O,\,6C{{O}_{2}}\] and:
A)
38 molecules of ATP
done
clear
B)
28 ATP
done
clear
C)
38 ADP
done
clear
D)
28 ADP
done
clear
View Answer play_arrow
question_answer 204) A characteristic feature of ovary of Brassica campestris is:
A)
presence of replum
done
clear
B)
axilc placentation
done
clear
C)
epigynous
done
clear
D)
multilocular nature
done
clear
View Answer play_arrow
question_answer 205) One of the characteristic of Hydra is:
A)
predation
done
clear
B)
metamerism
done
clear
C)
hibernation
done
clear
D)
mimicry
done
clear
View Answer play_arrow
question_answer 206) Where does exoerythrocytic cycle take place in life cycle of Plasmodium?
A)
RBC of human
done
clear
B)
Human liver
done
clear
C)
Stomach of Anopheles mosquito
done
clear
D)
Salivary and of Anopheles mosquito
done
clear
View Answer play_arrow
question_answer 207) Primary acceptor of \[C{{O}_{2}}\] in photosynthesis is:
A)
phosphoric acid
done
clear
B)
ribulose phosphate
done
clear
C)
glucose
done
clear
D)
ribulose 1, 5 biphosphate
done
clear
View Answer play_arrow
question_answer 208) If Hydra is cut transversely in three equal parts then:
A)
all three parts will die
done
clear
B)
regeneration will occur in all the three parts
done
clear
C)
regeneration will occur only in anterior
done
clear
D)
regeneration occurs only in middle part
done
clear
View Answer play_arrow
question_answer 209) Which of the following pair lack the unit membrane?
A)
Nucleus and ER
done
clear
B)
Mitochondria and chloroplast
done
clear
C)
Ribosome and nucleolus
done
clear
D)
Golgi body and lysosome
done
clear
View Answer play_arrow
question_answer 210) Which of the following statement is correct?
A)
In Cycas, megasporophyll produce pollen grains
done
clear
B)
In Agaricus gills produce basidiospores
done
clear
C)
In Aspergillus, fruiting body is perithecium
done
clear
D)
In Funaria, capsule represents gametophytic generation
done
clear
View Answer play_arrow
question_answer 211) The number of DNA in chromosome at G2 stage:
A)
one
done
clear
B)
two
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 212) How many egg are found in egg chamber of female Cockroach?
A)
2
done
clear
B)
4
done
clear
C)
8
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 213) Which of the following is a disaccharide?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Sucrose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 214) If all the peptide bonds of protein are broken, then the remaining part is :
A)
amide
done
clear
B)
oligosaccharide
done
clear
C)
polypeptide
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 215) Periplaneta has no respiratory pigment in its blood because:
A)
air is conducted directly to the body tissues
done
clear
B)
it has haemocoelom
done
clear
C)
it has anaerobic respiration
done
clear
D)
it lacks blood cell in blood
done
clear
View Answer play_arrow
question_answer 216) Which is correct for meiotic metaphase\[\text{I}\]?
A)
Bivalents arc arranged at equator
done
clear
B)
Univalents arc arranged at equator
done
clear
C)
Non-homologous chromosomes forms pair
done
clear
D)
Spindle fibres are attached at chromomere
done
clear
View Answer play_arrow
question_answer 217) Hydrolysis of lipid yields:
A)
fats
done
clear
B)
fatty acids and glycerol
done
clear
C)
mannose and glyccrol
done
clear
D)
maltose and fatty acid
done
clear
View Answer play_arrow
question_answer 218) Ctenophora shows affinities with:
A)
Cnidaria
done
clear
B)
Aschelminthes
done
clear
C)
Cephalopoda
done
clear
D)
Turbelaria
done
clear
View Answer play_arrow
question_answer 219) In the following how the sap wood is converted into heart wood?
A)
By degeneration of protoplast of living cells
done
clear
B)
Tylosis formation
done
clear
C)
By deposition of resins, oil, gums etc
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 220) In which of the following animal cleavage divisions arc restricted to a small part of cytoplasm and nucleus in animal pole of egg?
A)
Cockroach
done
clear
B)
Frog
done
clear
C)
Chick
done
clear
D)
Rabbit
done
clear
View Answer play_arrow
question_answer 221) Exception of Mendels law is:
A)
independent assortment
done
clear
B)
linkage
done
clear
C)
purity of gametes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 222) What type of gametes will form by genotype Rr Yy?
A)
RY, Ry, rY, ry
done
clear
B)
RY, Ry, ry, ry
done
clear
C)
Ry, Ry, Yy, ry
done
clear
D)
Rr, RR, Yy, YY
done
clear
View Answer play_arrow
question_answer 223) The position of protoxylem in leaf is:
A)
adaxial
done
clear
B)
abaxial
done
clear
C)
surrounded by metaxylem
done
clear
D)
lateral
done
clear
View Answer play_arrow
question_answer 224) In Pheretinia, locomotion occurs with the help of:
A)
circular muscles
done
clear
B)
longitudinal muscles and setae
done
clear
C)
circular, longitudinal muscles and setae
done
clear
D)
parapodia
done
clear
View Answer play_arrow
question_answer 225) The difference in phloem of gymnosperms and angiosperms is due to:
A)
parenchyma
done
clear
B)
sieve cell
done
clear
C)
companion cell
done
clear
D)
fibres
done
clear
View Answer play_arrow
question_answer 226) A type of life cycle in which plasmogamy, karyogamy, haplodization takes place but not at specific place in life cycle of an organism is called as:
A)
parasexuality
done
clear
B)
heterozygosity
done
clear
C)
homozygosity
done
clear
D)
asexuality
done
clear
View Answer play_arrow
question_answer 227) In Amorbn which controls the cytoplasmic osmality:
A)
nucleus
done
clear
B)
ectoplasm
done
clear
C)
biurets
done
clear
D)
contractile vacuole
done
clear
View Answer play_arrow
question_answer 228) Branched, aseptate, coenocytic mycelium present in:
A)
Aspergillus
done
clear
B)
Albugo
done
clear
C)
Penicillium
done
clear
D)
Erysiphe
done
clear
View Answer play_arrow
question_answer 229) In which of the following aestivation sepals/petals one margin covers the other and its margin is covered by previous one?
A)
valvate
done
clear
B)
twisted
done
clear
C)
imbricate
done
clear
D)
quincuncial
done
clear
View Answer play_arrow
question_answer 230) In Cockroach vision is due to:
A)
one compound eye
done
clear
B)
only two compound eyes
done
clear
C)
two simple eyes
done
clear
D)
two compound and two simple eyes
done
clear
View Answer play_arrow
question_answer 231) The economically important plant of Malvaceae:
A)
Gossypium hirstum
done
clear
B)
Hibiscus cannabis
done
clear
C)
Abelmoschus esculentum
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 232) When tall and dwarf plants are crossed, from which cross 1 : 1 ratio is obtained?
A)
Tt and tt
done
clear
B)
tt and tt
done
clear
C)
\[Tt\times Tt\]
done
clear
D)
TT and Tt
done
clear
View Answer play_arrow
question_answer 233) The cells without nuclei are present in:
A)
vascular cambium
done
clear
B)
root hair
done
clear
C)
companion cell
done
clear
D)
members of sieve tube
done
clear
View Answer play_arrow
question_answer 234) Which of the following is not correctly matched?
A)
Rhinoncephalon-olfactory
done
clear
B)
Hypothalamus-pituitary
done
clear
C)
Cerebellum-balance
done
clear
D)
Medulla oblongata-temperature regulation
done
clear
View Answer play_arrow
question_answer 235) Pyruvic acid is the end product of which process?
A)
Krebs cycle
done
clear
B)
Calvin cycle
done
clear
C)
Pentose phosphate pathway
done
clear
D)
Glycolysis
done
clear
View Answer play_arrow
question_answer 236) The nutritive layer of micros porangia of
A)
endothecium
done
clear
B)
exothecium
done
clear
C)
sporogenous tissue
done
clear
D)
tapetum
done
clear
View Answer play_arrow
question_answer 237) The scientist who was awarded Nobel prize in 1959 for in vitro synthesis of polyribonucleotide:
A)
Komberg
done
clear
B)
Calvin
done
clear
C)
Khurana
done
clear
D)
Ochoa
done
clear
View Answer play_arrow
question_answer 238) Which of the following have sunken stomata?
A)
Nerium
done
clear
B)
Manyfera
done
clear
C)
Hydrilla
done
clear
D)
Zea mays
done
clear
View Answer play_arrow
question_answer 239) Heterocercal tail is found in:
A)
Cartilaginous fishes
done
clear
B)
Bony fishes
done
clear
C)
Whale
done
clear
D)
Amphibians
done
clear
View Answer play_arrow
question_answer 240) When a plasmolysis cells is placed in a hypotonic solution then water will move inside the cell. Which force causes this?
A)
DPD
done
clear
B)
OF
done
clear
C)
WP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 241) Dormancy of seed is broken by:
A)
auxin
done
clear
B)
gibberellins
done
clear
C)
ethylene
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 242) Mitosis occurs in:
A)
haploid individuals
done
clear
B)
diploid individuals
done
clear
C)
in bacteria only
done
clear
D)
both a and b
done
clear
View Answer play_arrow
question_answer 243) Girth of dicot stem increases by the activity of:
A)
apical meristem
done
clear
B)
intercalary meristem
done
clear
C)
lateral meristem
done
clear
D)
procambium meristem
done
clear
View Answer play_arrow
question_answer 244) Group of two or more than two plant species is called as :
A)
plant community
done
clear
B)
animal ecosystem
done
clear
C)
Plant ecosystem
done
clear
D)
ecological niche
done
clear
View Answer play_arrow
question_answer 245) Which type of kidneys are found in amphibian?
A)
Holonephric
done
clear
B)
Mesonephric
done
clear
C)
Pronephric
done
clear
D)
Metanephric
done
clear
View Answer play_arrow
question_answer 246) That haploid cell which divides by mitosis to form embryo sac is :
A)
megaspore mother cell
done
clear
B)
microspore mother cell
done
clear
C)
functional megaspore
done
clear
D)
non-functional megaspore
done
clear
View Answer play_arrow
question_answer 247) One of the nucleotide of DNA:
A)
adenine
done
clear
B)
deoxyadenylic acid
done
clear
C)
adenosine
done
clear
D)
deoxyuridine phosphate
done
clear
View Answer play_arrow
question_answer 248) Which parts of cell have enzymes for glycolysis?
A)
Cytoplasm
done
clear
B)
Mitochondria
done
clear
C)
Golgi complex
done
clear
D)
Nucleus
done
clear
View Answer play_arrow
question_answer 249) Botanical name of species which cause white rust of Crucifers?
A)
Peronospora parasitica
done
clear
B)
Puccinia graminis
done
clear
C)
Pythium debargamim
done
clear
D)
Albugo Candida
done
clear
View Answer play_arrow
question_answer 250) What is haemozine?
A)
Undigested part of blood in trophozoite of Plasmodium
done
clear
B)
Blood pigment of Anopheles
done
clear
C)
Dcomposed blood in merozoites
done
clear
D)
Granules in the blood of infected person
done
clear
View Answer play_arrow
question_answer 251) Family Lilliaceae is characterized by:
A)
trimerous flower
done
clear
B)
tetramerous flower
done
clear
C)
pentamerous flower
done
clear
D)
zygomorphic flower
done
clear
View Answer play_arrow
question_answer 252) In sweet pea tendrils are modified:
A)
stipule
done
clear
B)
stem
done
clear
C)
leaf
done
clear
D)
leaflet
done
clear
View Answer play_arrow
question_answer 253) Root cap is absent in:
A)
mesophytes
done
clear
B)
hydrophytes
done
clear
C)
epiphytes
done
clear
D)
xerophytes
done
clear
View Answer play_arrow
question_answer 254) Which of the following nerve supplies organ of corti?
A)
Auditory
done
clear
B)
Olfactory
done
clear
C)
Trochlear
done
clear
D)
Vagus
done
clear
View Answer play_arrow
question_answer 255) Nucleic acid segement which is used to find the position of a gene and it forms a hybrid with this gene would be?
A)
Retrovirus
done
clear
B)
Probe
done
clear
C)
Vector
done
clear
D)
Clone
done
clear
View Answer play_arrow
question_answer 256) Insects eggs are :
A)
microlecithal and centrolecithal
done
clear
B)
megalecithal and isolecithal
done
clear
C)
megalecithal and centrolecithal
done
clear
D)
megalecithal and telolacithal
done
clear
View Answer play_arrow
question_answer 257) A pond is a:
A)
biome
done
clear
B)
natural ecosystem
done
clear
C)
artificial ecosystem
done
clear
D)
community of plants and animals
done
clear
View Answer play_arrow
question_answer 258) In crustaceans, respiration takes place by:
A)
gills
done
clear
B)
book lungs
done
clear
C)
ctenidia
done
clear
D)
trachea
done
clear
View Answer play_arrow
question_answer 259) Which of the two characters are typical of Ascaris ?
A)
Sexual dimorphism and rhabditiform larva
done
clear
B)
Hennaphroditism and pseudocoelomic
done
clear
C)
Parasitism and metamerism
done
clear
D)
Multilocular heart.
done
clear
View Answer play_arrow
question_answer 260) In a monohybrid cross when \[{{F}_{1}}\] is crossed with homozygous dominant parent then which type of offsprings will obtain?
A)
Dominant; recessive (3 : 1)
done
clear
B)
Only recessive
done
clear
C)
Dominant: recessive (1 : 1)
done
clear
D)
No recessive
done
clear
View Answer play_arrow
question_answer 261) Which one of the following body functions is not performed by kidneys?
A)
Excretion
done
clear
B)
Osmoregulation
done
clear
C)
Regulation of blood volume
done
clear
D)
Destruction of dead blood corpuscles
done
clear
View Answer play_arrow
question_answer 262) Which gland is associated with fur hairs of Rabbit?
A)
Mucous gland
done
clear
B)
Sweat gland
done
clear
C)
Mammary gland
done
clear
D)
Sebaceous gland
done
clear
View Answer play_arrow
question_answer 263) The study of fossils is called:
A)
Palynology
done
clear
B)
Palaeontology
done
clear
C)
Fossil systematic
done
clear
D)
Pharmticognosy
done
clear
View Answer play_arrow
question_answer 264) The body cavity of Ascaris is:
A)
lined by mesoderm
done
clear
B)
not lined by mesoderm
done
clear
C)
formed by octoderm
done
clear
D)
filled with parenchyma
done
clear
View Answer play_arrow
question_answer 265) The characteristic of blue-green algae is:
A)
DNA without histone
done
clear
B)
nuclear membrane absent
done
clear
C)
70S ribosomes
done
clear
D)
alt of the above
done
clear
View Answer play_arrow
question_answer 266) Classification of sponges is primariy based on the:
A)
body organization
done
clear
B)
body plan
done
clear
C)
skeleton
done
clear
D)
canal system
done
clear
View Answer play_arrow
question_answer 267) In mammals corpus Inuteum is found in which organ?
A)
Brain
done
clear
B)
Ovary
done
clear
C)
Liver
done
clear
D)
Eyes
done
clear
View Answer play_arrow
question_answer 268) Which type of rhizoids are present in Riccia?
A)
Unicellular smooth
done
clear
B)
Multicellular smooth
done
clear
C)
Unicellular smooth and tuberculated
done
clear
D)
Multicellular smooth and tuberculated
done
clear
View Answer play_arrow
question_answer 269) Which character is found only in mammals?
A)
Neck
done
clear
B)
Diaphragm
done
clear
C)
Optic lobes of brain
done
clear
D)
Tail
done
clear
View Answer play_arrow
question_answer 270) Number of ATP obtained at the end of Krebs cycle are:
A)
2 ATP
done
clear
B)
4 ATP
done
clear
C)
36 ATP
done
clear
D)
38 ATP
done
clear
View Answer play_arrow
question_answer 271) Which is correct about the bile of Rabbit?
A)
It is synthesized by gall bladder and also stored there
done
clear
B)
It is an enzyme which emulsify the fats
done
clear
C)
It contain bile salts and bile pigments
done
clear
D)
Bilirubin present in it dicomposes fats
done
clear
View Answer play_arrow
question_answer 272) Which type of vascular bundles are found in monocot stem?
A)
Coilateral, open, endarch
done
clear
B)
Radial, open, diarch
done
clear
C)
Radial, open, mesarch
done
clear
D)
Coliateral, closed, endarch
done
clear
View Answer play_arrow
question_answer 273) Which type of larva found in Hydra?
A)
Parenchymula
done
clear
B)
Amphiblastula
done
clear
C)
Trochopbore
done
clear
D)
Hydrula
done
clear
View Answer play_arrow
question_answer 274) In rhizome of Pteridium, stele which is composed of two or more than two concentric rings of vascular bundles is called:
A)
polycyclic
done
clear
B)
siphonostele
done
clear
C)
ectophloic siphonostele
done
clear
D)
cladosiphonostelo
done
clear
View Answer play_arrow
question_answer 275) Which of the following is not the correct for gastrulation?
A)
Archenteron is formed
done
clear
B)
all germinal layers are formed
done
clear
C)
Morphogenetic movements
done
clear
D)
Some blastomeres and blastoa degenerate
done
clear
View Answer play_arrow
question_answer 276) The chemical formula of starch is:
A)
\[{{\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{10}}}{{\text{O}}_{\text{5}}}\text{)}}_{\text{n}}}\]
done
clear
B)
\[{{\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{6}}\text{)}}_{\text{n}}}\]
done
clear
C)
\[{{\text{(}{{\text{C}}_{12}}{{\text{H}}_{\text{22}}}{{\text{O}}_{11}}\text{)}}_{\text{n}}}\]
done
clear
D)
\[\text{C}{{\text{H}}_{3}}\text{CO}{{\text{O}}_{11}}\text{H}\]
done
clear
View Answer play_arrow
question_answer 277) Which of the following mollusc is formed by a larva which have torsion?
A)
Lamelledens
done
clear
B)
Pila
done
clear
C)
Sepia
done
clear
D)
Octopus
done
clear
View Answer play_arrow
question_answer 278) At which stage of spermatogenesis sperm aquire their whole structural maturity and they contain a haploid nucleus and other organs:
A)
spermiogenesis
done
clear
B)
growth phase
done
clear
C)
multiplication phase
done
clear
D)
maturation phase
done
clear
View Answer play_arrow
question_answer 279) Which of the following is used during discovery of Calvin cycle?
A)
Spirogyra
done
clear
B)
Volvox
done
clear
C)
Chlamydomonas
done
clear
D)
Chlorella
done
clear
View Answer play_arrow
question_answer 280) What happens in anterior part of Amoeba at the tune of formation of pseudopodia?
A)
plasma gel convert into plasma sol
done
clear
B)
plasma sol convert into plasma gel
done
clear
C)
ectoplasm convert into endoplasm
done
clear
D)
endoplasm convert into ectoplasm
done
clear
View Answer play_arrow
question_answer 281) Respiratory quotient of which diet is less than unity?
A)
Carbohydrate
done
clear
B)
Fats
done
clear
C)
Organic acid
done
clear
D)
Sugar
done
clear
View Answer play_arrow
question_answer 282) In Rabbit at the time of fertilization zygote is formed in :
A)
coelom
done
clear
B)
fallopian tube
done
clear
C)
uterus
done
clear
D)
vagina
done
clear
View Answer play_arrow
question_answer 283) Velamen and spongy tissue is found in:
A)
breathing roots
done
clear
B)
parasitic roots
done
clear
C)
tuberous roots
done
clear
D)
epiphytic roots
done
clear
View Answer play_arrow
question_answer 284) The function of clitellum in Pheretima is:
A)
formation of cocoon
done
clear
B)
secretion of hormone
done
clear
C)
nutrition of sperm
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 285) Which animal is protochordate?
A)
Herdmania
done
clear
B)
Balanoglossus
done
clear
C)
Branchiostoma
done
clear
D)
Botryllus
done
clear
View Answer play_arrow
question_answer 286) Which of the following is not a function of water in cell ?
A)
It provides energy for chemical reaction
done
clear
B)
It act as a solvent
done
clear
C)
It provides a medium for chemical reaction
done
clear
D)
It release hydrogen ions on ionization
done
clear
View Answer play_arrow
question_answer 287) In life cycle of Plasmodium exflagellation leads to:
A)
formation of microgametes
done
clear
B)
formation of flagella in sperm
done
clear
C)
destruction of flagella from sperm
done
clear
D)
formation of sporozoites
done
clear
View Answer play_arrow
question_answer 288) In which of the following reptiles four chambered heart is present?
A)
Lizard
done
clear
B)
Snake
done
clear
C)
Scorpion
done
clear
D)
Crocodile
done
clear
View Answer play_arrow
question_answer 289) Rate of transpiration is measured by:
A)
manometer
done
clear
B)
auxanometer
done
clear
C)
potometer
done
clear
D)
barometer
done
clear
View Answer play_arrow
question_answer 290) Pigments present in chloroplast of Ulothrix :
A)
chlorophyll a, chl-b, fucoxanthin \[\text{-}\]carotene
done
clear
B)
chl-a, chl-b, chl-c, C-phycocyanin, C-phycoerythrin
done
clear
C)
chl-a, chl-b. \[\text{-}\]carotene, xanthophyll
done
clear
D)
chl-a, chl-b, r-phycocyanine r-phycoerythrin
done
clear
View Answer play_arrow
question_answer 291) In Mirabilis a hybrid for red (RR) and white (rr) flower produces pink (Rr) flower. A plant with pink flower is crossed with white flower the expected phenotypic ratio is:
A)
red: pink : white (1 : 2 :1)
done
clear
B)
pink: white (1 : 1)
done
clear
C)
red: pink (1 : 1)
done
clear
D)
red: white (3 : 1)
done
clear
View Answer play_arrow
question_answer 292) Which one hormone of the pituitary of the Rabbit controls the protein metabolism and growth of skeleton?
A)
lodo thyroxine
done
clear
B)
Leutotrophic hormone
done
clear
C)
Somatotrophic hormone
done
clear
D)
Oxytosine
done
clear
View Answer play_arrow
question_answer 293) Which of the following is the site of lipid synthesis?
A)
Rough ER
done
clear
B)
Smooth ER
done
clear
C)
Golgi bodies
done
clear
D)
Ribosome
done
clear
View Answer play_arrow
question_answer 294) Which of the following vessel in Rabbit starts with capillaries and ends in capillaries?
A)
Pulmonary artery
done
clear
B)
Renal vein
done
clear
C)
Hepatic portal vein
done
clear
D)
Renal artery
done
clear
View Answer play_arrow
question_answer 295) Which character is not same in Aves and Mammals?
A)
Single systemic arch
done
clear
B)
Metanephric kidney
done
clear
C)
Seven cervical vertebrae
done
clear
D)
Homeotherms
done
clear
View Answer play_arrow
question_answer 296) What is pollen grain?
A)
Microspore mother cell
done
clear
B)
Male gamete
done
clear
C)
Male gametophyte
done
clear
D)
Partially developed embryo
done
clear
View Answer play_arrow
question_answer 297) Solenocytes and nephridia are respectively found in:
A)
PIatyhelminth and Annelids
done
clear
B)
Annelids and Nematoda
done
clear
C)
Cendaria and Mollusca
done
clear
D)
Mollusca and Echinodermata
done
clear
View Answer play_arrow
question_answer 298) If a cell shrinks when placed in a solution this solution is:
A)
hypotonic
done
clear
B)
hypertonic
done
clear
C)
isotonic
done
clear
D)
mesotonic
done
clear
View Answer play_arrow