question_answer 1) In an adiabatic process relation of relative change in pressure \[\frac{\Delta P}{P}\]with relative change m volume\[\frac{\Delta P}{V}\] is:
A)
\[\frac{1}{\gamma }\left( \frac{\Delta V}{V} \right)\]
done
clear
B)
\[\frac{1}{{{\gamma }^{2}}}\left( \frac{\Delta V}{V} \right)\]
done
clear
C)
\[-\gamma \left( \frac{\Delta V}{V} \right)\]
done
clear
D)
\[\gamma \left( \frac{\Delta V}{V} \right)\]
done
clear
View Answer play_arrow
question_answer 2) There is a rough black spot on a polished surface. It is heated to a temperature \[1400{}^\circ C\] then:
A)
black spot will be seen more bright
done
clear
B)
spot and plate will be seen with equal brightness
done
clear
C)
neither spot nor plate will be seen bright
done
clear
D)
polished surface will be seen more bright
done
clear
View Answer play_arrow
question_answer 3) A body cool from \[{{80}^{o}}C\]to \[{{70}^{o}}C\] in 6 minutes. Then in cooling from \[{{70}^{o}}C\] to \[{{60}^{o}}C\], time taken will be:
A)
less than 6 minutes
done
clear
B)
6 minutes
done
clear
C)
mere than 6 minutes
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 4) A hammer of mass 1 kg having speed of\[50\text{ }m/s,\] hit a iron nail of mass \[200gm\]. If specific heat of iron is \[0.105cal/g{{m}^{o}}C\]and half the energy is converted into heat, the raise in temperature of nail is:
A)
\[{{7.1}^{o}}C\]
done
clear
B)
\[{{9.2}^{o}}C\]
done
clear
C)
\[{{10.5}^{o}}C\]
done
clear
D)
\[{{12.1}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 5) Thickness of a glass plate is t and its refractive index is n. Velocity of light in vacuum is c. Then minimum time taken by light in passing through the glass plate is:
A)
\[\frac{ct}{n}\]
done
clear
B)
\[\frac{nt}{c}\]
done
clear
C)
\[nct\]
done
clear
D)
\[\frac{nc}{t}\]
done
clear
View Answer play_arrow
question_answer 6) There are some points in stationary wave which:
A)
are never at rest
done
clear
B)
are always in motion
done
clear
C)
are at rest twice in each cycle
done
clear
D)
are at rest once in each cycle
done
clear
View Answer play_arrow
question_answer 7) A closed pipe of length L resonate with a lulling fork. The length of open pipe to resonate with the same tuning fork will be:
A)
\[\frac{L}{2}\]
done
clear
B)
\[L\]
done
clear
C)
\[\frac{3L}{2}\]
done
clear
D)
\[2L\]
done
clear
View Answer play_arrow
question_answer 8) Length of a piano wire is \[1.1\,m\]and its mass is \[160g\]. If its frequency is \[33Hz\], then tension in wire in newton is:
A)
\[556\,N\]
done
clear
B)
\[~635\,N\]
done
clear
C)
\[766\,N\]
done
clear
D)
\[995\,N\]
done
clear
View Answer play_arrow
question_answer 9) Two sound sources \[{{S}_{1}}\] and \[{{S}_{2}}\] of frequencies \[324Hz\]and \[320Hz\] are placed at certain separation. An observer is moving on line joining them. If he hears no beats then speed of observer is: \[(\upsilon =344m/\sec )\]
A)
\[20m/s\]
done
clear
B)
\[10m/s\]
done
clear
C)
\[5m/s\]
done
clear
D)
\[3.1m/s\]
done
clear
View Answer play_arrow
question_answer 10) A car moving with speed \[28m/s\]sound a horn of frequency\[500Hz\], passes another car moving with a speed of \[13m/s\]in same direction, then frequency of horn heard by driver of another car is (speed of sound is \[342m/s\]):
A)
\[480\,Hz\]
done
clear
B)
\[500\,Hz\]
done
clear
C)
\[520\,Hz\]
done
clear
D)
\[580\,Hz\]
done
clear
View Answer play_arrow
question_answer 11) Ratio of energies of two photons whose wavelengths are \[6000\overset{\text{o}}{\mathop{\text{A}}}\,\]and \[4000\overset{\text{o}}{\mathop{\text{A}}}\,\] is:
A)
\[2:3\]
done
clear
B)
\[3:2\]
done
clear
C)
\[1:5\]
done
clear
D)
\[5:1\]
done
clear
View Answer play_arrow
question_answer 12) Behaviour of zone plate coincides with:
A)
prism
done
clear
B)
grating
done
clear
C)
biprism
done
clear
D)
lens
done
clear
View Answer play_arrow
question_answer 13) Two charges\[4q\]and\[q\] are placed at a distance\[I\] another charge Q is placed at the midpoint of line joining the charges\[4q\]and\[q\] If net force on charge\[q\] is zero, then value of \[Q\] is:
A)
\[+q\]
done
clear
B)
\[-q\]
done
clear
C)
\[+2q\]
done
clear
D)
\[3q\]
done
clear
View Answer play_arrow
question_answer 14) Potential on the surface of a hollow sphere is 10 volt. Then potential at its centre will be:
A)
\[10\,volt\]
done
clear
B)
\[5\,volt\]
done
clear
C)
\[25\,volt\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 15) A charge is placed on the axis of a dipole at a distance r from centre then charge experiences a force F. If distance from the centre is doubled then new value of force will be:
A)
zero
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{F}{4}\]
done
clear
D)
\[\frac{F}{8}\]
done
clear
View Answer play_arrow
question_answer 16) Ratio of gravitational force and electrostatic force between two electrons is:
A)
\[{{10}^{-36}}\]
done
clear
B)
\[{{10}^{-39}}\]
done
clear
C)
\[{{10}^{-42}}\]
done
clear
D)
\[{{10}^{-47}}\]
done
clear
View Answer play_arrow
question_answer 17) In a coil current changes from \[4A\]to \[2A\] in\[0.05\text{ }sec,\] if induced e.m.f. is 8 volt then self-inductance of the coil is:
A)
\[0.5H\]
done
clear
B)
\[0.35H\]
done
clear
C)
\[0.2H\]
done
clear
D)
\[2mH\]
done
clear
View Answer play_arrow
question_answer 18) Dimension of a rectangular coil is\[20cm\times 10cm\]. Number of turns in coil is 60. It is rotating in an uniform magnetic field of \[0.5T,\]with a speed 1800 r.p.m. the maximum induced e.m.f. is:
A)
\[98\,V\]
done
clear
B)
\[110\,V\]
done
clear
C)
\[113\,V\]
done
clear
D)
\[118\,V\]
done
clear
View Answer play_arrow
question_answer 19) In a stop up transformer ratio of number of turn in primary to secondary coil is \[1:25\]. If input voltage is \[230\text{ }volt\]and load current is\[2amp\], then current in primary coil is:
A)
\[50A\]
done
clear
B)
\[25A\]
done
clear
C)
\[12.5A\]
done
clear
D)
\[6.25A\]
done
clear
View Answer play_arrow
question_answer 20) Value of current in an A.C. circuit is \[I=2\cos \omega t+\theta ),\]then the value of\[{{I}_{rms}}\]is:
A)
\[\sqrt{\text{2}}\text{A}\]
done
clear
B)
\[\frac{\text{1}}{\sqrt{\text{2}}}\text{A}\]
done
clear
C)
\[\text{2A}\]
done
clear
D)
\[\frac{\text{1}}{2}\text{A}\]
done
clear
View Answer play_arrow
question_answer 21) Impedence of a coil is \[100ohm\]at frequency \[50Hz\], then its impedence on \[150Hz\]is:
A)
\[100\,ohm\]
done
clear
B)
\[300\,ohm\]
done
clear
C)
\[450\,ohm\]
done
clear
D)
\[600\,ohm\]
done
clear
View Answer play_arrow
question_answer 22) For a paramagnetic substance suspectibility is related with temperature as:
A)
\[V\propto T\]
done
clear
B)
\[X\propto \frac{1}{T}\]
done
clear
C)
\[X\propto \frac{1}{T-T}\]
done
clear
D)
constant
done
clear
View Answer play_arrow
question_answer 23) Kinetic energy of an electron in hydrogen atom in Bohrs orbit of radius r:
A)
\[\frac{KE}{2r}\]
done
clear
B)
\[\frac{K{{E}^{2}}}{2r}\]
done
clear
C)
\[\frac{KE}{r}\]
done
clear
D)
\[\frac{2KE}{r}\]
done
clear
View Answer play_arrow
question_answer 24) Wavelength of second line of Lyman series is \[1025\overset{\text{o}}{\mathop{\text{A}}}\,,\] then wavelength of first line is:
A)
\[825\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[900\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1215\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1325\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 25) In an atom quantum number in a orbit n, \[l\] and m are same. The maximum number of electrons are:
A)
\[2\]
done
clear
B)
\[2{{n}^{2}}\]
done
clear
C)
\[(2l+1)\]
done
clear
D)
\[2(2l+1)\]
done
clear
View Answer play_arrow
question_answer 26) Acceleration voltage in an electron gun is 50000 volt, de-Brogile wavelength of electron will be:
A)
\[0.55\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.055\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.077\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.095\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 27) Momentum of X-ray photon of wavelength \[0.01\overset{\text{o}}{\mathop{\text{A}}}\,\]in kg-m/sec is:
A)
\[6.6\times {{10}^{-22}}\]
done
clear
B)
\[6.6\times {{10}^{-32}}\]
done
clear
C)
\[6.6\times {{10}^{-46}}\]
done
clear
D)
\[6.6\times {{10}^{-27}}\]
done
clear
View Answer play_arrow
question_answer 28) Work function of metal is\[2.4\,eV\]. Then for photo electorons omission, maximum wavelength of photon will be:
A)
\[3000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[3500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[5000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 29) Ratio of ionization power of \[\alpha \] and \[\]particles emitted from a radioactive element is;
A)
\[1:100\]
done
clear
B)
\[100:1\]
done
clear
C)
\[1000:1\]
done
clear
D)
\[1:1000\]
done
clear
View Answer play_arrow
question_answer 30) The half-life of radioactive clement is 5 days, then time taken by in decaying \[\frac{\text{7}}{\text{8}}\text{th}\] of its initial amount:
A)
\[2.5\,day\]
done
clear
B)
\[5\,day\]
done
clear
C)
\[10\,day\]
done
clear
D)
\[15\,day\]
done
clear
View Answer play_arrow
question_answer 31) \[_{\text{90}}\text{T}{{\text{h}}^{\text{232}}}\]disintegrate continuously and finally converts into\[_{\text{82}}\text{P}{{\text{b}}^{\text{208}}}\text{,}\]then number of \[\alpha \] and \[\] particles produced are respectively:
A)
\[4,6~\]
done
clear
B)
\[6,4\]
done
clear
C)
\[3,2\]
done
clear
D)
\[2,3\]
done
clear
View Answer play_arrow
question_answer 32) In a diode thermionic emission from cathode will be maximum when cathode has:
A)
high work function, low temperature
done
clear
B)
high work function, high temperature
done
clear
C)
low work function, high temperature
done
clear
D)
low work function, low tern temperature
done
clear
View Answer play_arrow
question_answer 33) For a triode \[\mu =25,Rp=40\,k\Omega \] \[{{R}_{L}}=10\,k\Omega \]and input signal voltage is 0.5 volt. Then output voltage is:
A)
\[1.25\,volt\]
done
clear
B)
\[2.5\,volt\]
done
clear
C)
\[5\,volt\]
done
clear
D)
\[10\,volt\]
done
clear
View Answer play_arrow
question_answer 34) Which of the following is used as an impurity for p-type semiconductor?
A)
Boran
done
clear
B)
Bismuth
done
clear
C)
Arsenic
done
clear
D)
Phosphorous
done
clear
View Answer play_arrow
question_answer 35) When a plane mirror is placed horizontally on a level ground at a distance of 60 metre from the foot of a tower. The top of the tower and its image in the mirror subtend at the eye an angle of \[{{90}^{o}}\], the height of the tower is:
A)
\[30\,m\]
done
clear
B)
\[40\,m\]
done
clear
C)
\[60\,m\]
done
clear
D)
\[90\,m\]
done
clear
View Answer play_arrow
question_answer 36) Moment of inertia of a fan is\[0.06kg-{{m}^{2}}\]. If its angular speed is increased from 0 to 5 revolution/sec. Then its maximum angular momentum in M.K.S. unit is:
A)
\[0.3\,\pi \]
done
clear
B)
\[0.6\,\pi \]
done
clear
C)
\[\frac{0.03}{\,\pi }\]
done
clear
D)
\[\frac{0.6}{\,\pi }\]
done
clear
View Answer play_arrow
question_answer 37) A rigid body is rotating with an angular velocity\[\vec{\omega },\] torque acting on it is\[\vec{t}\]and an angular momentum is\[\vec{L}.\] Then relation between\[\vec{t}\] and\[\vec{L}\] is :
A)
\[\vec{t}=\vec{\omega }\times \vec{L}\]
done
clear
B)
\[\vec{t}=\vec{L}\frac{d\vec{\omega }}{dt}\]
done
clear
C)
\[\vec{t}=\vec{\omega }\frac{d\vec{L}}{dt}\]
done
clear
D)
\[\vec{t}=\frac{dL}{dt}\]
done
clear
View Answer play_arrow
question_answer 38) A block slides on inclined plane then velocity at the bottom of plane is v. If a ring roll on same inclined plane then its velocity at the bottom of plane is :
A)
\[2\upsilon \]
done
clear
B)
\[\upsilon \]
done
clear
C)
\[\frac{\upsilon }{2}\]
done
clear
D)
\[\frac{\upsilon }{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 39) A particle executes S.H.M. with an amplitude 4 cm. The displacement where its energy is half K.E. and half HE. is:
A)
\[2\sqrt{2}\]cm
done
clear
B)
2 cm
done
clear
C)
\[\sqrt{2}\]cm
done
clear
D)
\[\frac{3}{\sqrt{2}}\]cm
done
clear
View Answer play_arrow
question_answer 40) A body of mass in is taken from earth surface to height equal to earth radius R. Then change in its potential energy is:
A)
\[\frac{mgR}{2}\]
done
clear
B)
\[mgR\]
done
clear
C)
\[2mgR\]
done
clear
D)
\[\frac{mgR}{4}\]
done
clear
View Answer play_arrow
question_answer 41) A satellite is revolving close to surface of planet of radius R, its time period is T- Time period of satellite for a planet of radius 3R will be:
A)
\[\text{3}\sqrt{\text{3}}\text{T}\]
done
clear
B)
\[\frac{\sqrt{\text{3}}}{2}\text{T}\]
done
clear
C)
\[\frac{1}{3\sqrt{\text{3}}}\text{T}\]
done
clear
D)
\[\frac{1}{2}\text{T}\]
done
clear
View Answer play_arrow
question_answer 42) A second pendulum is suspended in satellite revolving round the earth a height 2R above earth surface, then its time period in second is:
A)
zero
done
clear
B)
2 sec
done
clear
C)
4 sec
done
clear
D)
infinite (\[\infty \])
done
clear
View Answer play_arrow
question_answer 43) A soap bubble is expand from radius 1 cm to 2 cm. If surface tension of soap solution is 0.05 N/m- The work done in joule will be:
A)
\[1.5\,\times {{10}^{-4}}\]
done
clear
B)
\[1.2\pi \times {{10}^{-4}}\]
done
clear
C)
\[0.3\pi \times {{10}^{-4}}\]
done
clear
D)
\[0.6\pi \times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 44) The height of liquid rise in a capillary tube will be:
A)
maximum at \[{{4}^{o}}C\]
done
clear
B)
maximum at \[{{0}^{o}}C\]
done
clear
C)
minimum at \[{{4}^{o}}C\]
done
clear
D)
minimum at \[{{0}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 45) Specific heat of gas at constant volume is:
A)
\[\frac{R}{(\gamma -1)}\]
done
clear
B)
\[\frac{R}{(\gamma +1)}\]
done
clear
C)
\[\frac{R}{\gamma }\]
done
clear
D)
\[R(\gamma -1)\]
done
clear
View Answer play_arrow
question_answer 46) A body radiate \[Q\text{ }cat/c{{m}^{2}}\]heat at a temperature\[{{227}^{o}}C\]. The rate of energy radiate at a temperature \[{{727}^{o}}C\]is (in\[cal/c{{m}^{2}}\]):
A)
\[2Q\]
done
clear
B)
\[4Q\]
done
clear
C)
\[16Q\]
done
clear
D)
\[32Q\]
done
clear
View Answer play_arrow
question_answer 47) A train of mass \[5\times {{10}^{6}}kg\]is moving with a speed of\[72\text{ }km/hr\]. By applying break it is stopped, then heat produced in kilo calories is:
A)
\[1.2\times {{10}^{5}}kcal\]
done
clear
B)
\[2.4\times {{10}^{5}}kcal\]
done
clear
C)
\[4.8\times {{10}^{5}}kcal\]
done
clear
D)
\[6\times {{10}^{5}}kcal\]
done
clear
View Answer play_arrow
question_answer 48) Refractive index of air is 1.003. The correct thickness of air column which will have one or more wavelength of yellow light \[(6000\overset{\text{o}}{\mathop{\text{A}}}\,)\] than in the same thickness in vacuum is:
A)
\[2mm\]
done
clear
B)
\[2cm\]
done
clear
C)
2m
done
clear
D)
\[2km\]
done
clear
View Answer play_arrow
question_answer 49) Length of a sonometer wire is \[l\]and its tension is T. If tension is made half, then its second harmonics is equal to first harmonics. Then the length of wire will be:
A)
\[\frac{l}{\sqrt{2}}\]
done
clear
B)
\[\frac{l}{2}\]
done
clear
C)
\[l\]
done
clear
D)
\[\frac{3l}{2}\]
done
clear
View Answer play_arrow
question_answer 50) Two sources of sound of same frequency are situated at a distance 1 metre apart. An observer is moving parallel to line joining them at a distance 10 metre. If distance between two consecutive an tinode is observed to be \[1.1\] metre, then frequency of source is (\[\upsilon \] = 300 m/sec):
A)
\[1000\,Hz\]
done
clear
B)
\[3000\,Hz\]
done
clear
C)
\[3500\,Hz\]
done
clear
D)
\[4000\,Hz\]
done
clear
View Answer play_arrow
question_answer 51) Two waves of intensity \[I\]and\[4I\] super impose on each other. Then in interference maximum and minimum intensity are respectively:
A)
\[3I\]and\[2I\]
done
clear
B)
\[25I\] and \[9I\]
done
clear
C)
\[9I\] and \[I\]
done
clear
D)
\[5I\] and \[3I\]
done
clear
View Answer play_arrow
question_answer 52) In Youngs double slit experiment distance between slit is 0.1mm and distance between slit and screen is\[20cm\]. If \[\lambda =5460\,\overset{\text{o}}{\mathop{\text{A}}}\,,\], then fringe width is:
A)
\[0.5mm\]
done
clear
B)
\[1.1\text{ }mm\]
done
clear
C)
\[1.5\text{ }mm\]
done
clear
D)
\[2.2mm\]
done
clear
View Answer play_arrow
question_answer 53) Five balls \[1,2,3,4,5\]are suspended side by side. The pair of balls \[(1,2),\] \[(1,4)\] and \[(2,5)\] attract each other whereas balls \[(2,3)\] and \[(4,5)\] repel each other. The ball number 1 must be:
A)
positively charged
done
clear
B)
negatively charged
done
clear
C)
neutral
done
clear
D)
conductor
done
clear
View Answer play_arrow
question_answer 54) A charger\[q\] is placed at the centre of circle of radius r. Then work done by charge Q in completing one round is:
A)
\[\frac{Q}{4\pi {{\varepsilon }_{0}}r}\]
done
clear
B)
\[\frac{Qq}{4\pi {{\varepsilon }_{0}}r}\]
done
clear
C)
zero
done
clear
D)
\[\frac{2q}{2r}\]
done
clear
View Answer play_arrow
question_answer 55) Two charge each of value \[-q\] are placed at \[(0\pm \,a)\]. A charge + Q is placed on the y-axis, then:
A)
it will execute S.H.M.
done
clear
B)
its motion is oscillatory, but not S.H.M.
done
clear
C)
it will remain stationary
done
clear
D)
will move along x-axis
done
clear
View Answer play_arrow
question_answer 56) Radius of a current carrying circular coil is R at a distance\[x(x>>R)\] on the axis of circular coil the magnetic field produced B is proportional to:
A)
\[B\propto \frac{1}{{{x}^{3/2}}}\]
done
clear
B)
\[B\propto \frac{1}{{{x}^{2}}}\]
done
clear
C)
\[B\propto \frac{1}{{{x}^{3}}}\]
done
clear
D)
\[B\propto \frac{1}{{{x}^{-1/2}}}\]
done
clear
View Answer play_arrow
question_answer 57) Atomic weight of single charge \[Li\] is \[6.06\,amu\]and its energy is\[400\,eV\]. It enter normally to a uniform magnetic field of \[0.8\] Tesla, then radius of circular path is:
A)
\[8.35\,cm\]
done
clear
B)
\[9.23\,cm\]
done
clear
C)
\[10.5\,cm\]
done
clear
D)
\[11.25\,cm\]
done
clear
View Answer play_arrow
question_answer 58) The length of a coil is \[0.3\,m\]and the number of turns is 2000. The area of cross-section of the coil is\[1.2\times {{10}^{-3}}{{m}^{2}}\]. Another coil is 300 turns is wrapped over the above coil. A current of 2A is passed through the solenoid and its direction is changed in\[0.25\,sec\]. Then the e.m.f induced in volt will be:
A)
\[7.6\times {{10}^{-2}}\]
done
clear
B)
\[4.8\times {{10}^{-3}}\]
done
clear
C)
\[3.2\times {{10}^{-4}}\]
done
clear
D)
\[3.2\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 59) Number of turns in primary coil of a transformer is 240. When input voltage is 20 volt, then output voltage is 2.5 volt. The number of turns in secondary coil is :
A)
250
done
clear
B)
100
done
clear
C)
10
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 60) When 4 amp current flows through a motor then power consumed is 20 watt, and resultant potential difference is 220 volt. The applied e.m.f. is :
A)
220 volt
done
clear
B)
225 volt
done
clear
C)
240 volt
done
clear
D)
260 volt
done
clear
View Answer play_arrow
question_answer 61) For ferromagnetic material:
A)
permeability is very high and suspectibility is positive
done
clear
B)
permeability is very high and suspectibility is negative
done
clear
C)
permeability is very low and suspectibility is positive
done
clear
D)
permeability is very low and suspectibility is negative
done
clear
View Answer play_arrow
question_answer 62) Angular momentum of an electron revolving in \[{{\text{2}}^{\text{nd}}}\] orbit of radius r:
A)
\[\frac{h}{2\pi }\]
done
clear
B)
\[\frac{h}{\pi }\]
done
clear
C)
\[\frac{h}{\pi r}\]
done
clear
D)
\[\frac{h}{2\pi r}\]
done
clear
View Answer play_arrow
question_answer 63) The ionization energy of hydrogen atom in excited state is:
A)
\[13.1eV\]
done
clear
B)
\[3.4eV\]
done
clear
C)
\[1.51eV\]
done
clear
D)
\[1.9eV\]
done
clear
View Answer play_arrow
question_answer 64) Minimum wavelength of X-ray? Is \[0.1\,\overset{\text{o}}{\mathop{\text{A}}}\,\], then applied accelerating voltage on the tube is:
A)
\[1.25\times {{10}^{3}}volt\]
done
clear
B)
\[1.24\times {{10}^{4}}volt\]
done
clear
C)
\[1.24\times {{10}^{5}}volt\]
done
clear
D)
\[1.24\times {{10}^{6}}volt\]
done
clear
View Answer play_arrow
question_answer 65) In X-ray spectra wavelength of \[{{k}_{\alpha }}\] line is \[\lambda ,\]depends upon atomic number of target Z as:
A)
\[\lambda \propto {{Z}^{2}}\]
done
clear
B)
\[\lambda \propto {{(Z-1)}^{2}}\]
done
clear
C)
\[\lambda \propto \frac{1}{(Z-1)}\]
done
clear
D)
\[\lambda \propto \frac{1}{{{(Z-1)}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 66) Mean mass of an element is 39.1 amu, The masses of its isotopes are 39, 40, 41 amu. If the abundance isotope of 40 amu is 0.012%, then relative abundance of other two is:
A)
93.7, 6.233
done
clear
B)
90.237, 8.751
done
clear
C)
93.073, 6.915
done
clear
D)
91.387, 8.603
done
clear
View Answer play_arrow
question_answer 67) In triode amplification voltage gain depends on:
A)
\[\mu ,RL\]and input voltage
done
clear
B)
\[{{R}_{P,}}{{R}_{L}}\]and \[\mu \]
done
clear
C)
\[\mu {{R}_{P,}}\] and input voltage
done
clear
D)
\[{{R}_{P,}}\mu \]and\[{{g}_{m}}\]
done
clear
View Answer play_arrow
question_answer 68) Forbidden energy gap in insulator is of:
A)
0 eV
done
clear
B)
0.7 eV
done
clear
C)
1.1 eV
done
clear
D)
5 eV
done
clear
View Answer play_arrow
question_answer 69) For a reverse biase \[\text{p-n}\] junction:
A)
p region is positive and the current is due to electrons
done
clear
B)
p region is positive and the current is duo to holes
done
clear
C)
p region is negative and the current is due to electrons
done
clear
D)
p region is negative and the current is due to both electrons and holes
done
clear
View Answer play_arrow
question_answer 70) In an X-ray tube, electrons are accelerated by a potential difference V, the minimum wavelength of emitted X-rays is:
A)
\[\frac{12400}{V}m\]
done
clear
B)
\[\frac{12400}{V}\overset{0}{\mathop{\text{A}}}\,\]
done
clear
C)
\[\frac{1.24}{V}\overset{0}{\mathop{\text{A}}}\,\]
done
clear
D)
\[\frac{V}{12400}\overset{0}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following is best absorbed of X-rays?
A)
Berylium
done
clear
B)
Copper
done
clear
C)
Gold
done
clear
D)
Lead
done
clear
View Answer play_arrow
question_answer 72) In electromagnet core is made of soft iron because:
A)
its suspectibility and permeability are high
done
clear
B)
suspectibility is high and permeability is low
done
clear
C)
both suspectibility and permeability are low
done
clear
D)
suspectibility is low and permeability are high
done
clear
View Answer play_arrow
question_answer 73) Two -spheres (A and B) each of mass 5 kg are connected by a light rod of length 1 m. Treating spheres as point. The ratio of moment of inertias about X axis perpendicular to length of rod and passing through centre of A and centre of rod is:
A)
1 : 1
done
clear
B)
4 : 1
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 74) The moment of inertia of a ring about its own axis is \[I.\] Then its moment of inertia, about a tangent in the plane is:
A)
\[\frac{I}{2}\]
done
clear
B)
\[\frac{3I}{2}\]
done
clear
C)
\[2I\]
done
clear
D)
\[\frac{5I}{2}\]
done
clear
View Answer play_arrow
question_answer 75) Mass of a ring is M and its radius is R. It is rotating about its own axis with angular velocity \[\omega \]. Two particles each of mass m sticks to the rim at diameter opposite point. Now angular velocity of ring is:
A)
\[\frac{M\omega }{2m}\]
done
clear
B)
\[\frac{2m\omega }{M}\]
done
clear
C)
\[\frac{(M+2m)\omega }{M}\]
done
clear
D)
\[\frac{M\omega }{(M+2m)}\]
done
clear
View Answer play_arrow
question_answer 76) A thin rod of mass M acts as a compound pendulum. Distance between centre of suspension and centre of mass is d. Moment of inertia about axis of suspension of\[I.\]Then distance between centre of suspension and centre of oscillation is:
A)
\[\frac{I}{Md}\]
done
clear
B)
\[\frac{I}{2Md}\]
done
clear
C)
\[\frac{I}{12Md}\]
done
clear
D)
\[\frac{3I}{4Md}\]
done
clear
View Answer play_arrow
question_answer 77) The following bodies have same mass, then which will have maximum moment of inertia about an axis perpendicular to plane and passing through centre of mass:
A)
ring of radius a
done
clear
B)
disc of radius a
done
clear
C)
square plate of side 2n
done
clear
D)
four rods forming a square of each side 2a
done
clear
View Answer play_arrow
question_answer 78) Length of a simple pendulum is \[I\]and its maximum angular displacement is \[\theta \] then its maximum K.E. is :
A)
\[mgl\,\sin \,\theta \]
done
clear
B)
\[mgl(1+\,\sin \,\theta )\]
done
clear
C)
\[mgl(1+\,\cos \,\theta )\]
done
clear
D)
\[mgl(1-\,\cos \,\theta )\]
done
clear
View Answer play_arrow
question_answer 79) Extension in length of a spring is 12 cm, when 5 kg mass is suspended on it. If spring oscillate vertically, then its time period is:
A)
0.7 sec
done
clear
B)
0.9 sec
done
clear
C)
1.1 sec
done
clear
D)
1.4 sec
done
clear
View Answer play_arrow
question_answer 80) \[n\] bullets each of mass \[m\] moving with velocity \[u\] strike normally on a wall. The collision is elastic then force on wall is:
A)
\[mnu\]
done
clear
B)
\[2\,mnu\]
done
clear
C)
\[4\,mnu\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 81) A planet revolve round the sun in elliptical orbit when planet is nearest to sun at a distance \[a\] its velocity is \[\upsilon \] and when it is farthest from sun at a distance \[b,\] its velocity is:
A)
\[\frac{b}{a}{{\upsilon }_{1}}\]
done
clear
B)
\[\left( \frac{a}{b} \right){{\upsilon }_{1}}\]
done
clear
C)
\[\left( \frac{\sqrt{a}}{b} \right){{\upsilon }_{1}}\]
done
clear
D)
\[\frac{\sqrt{b}}{a}{{\upsilon }_{1}}\]
done
clear
View Answer play_arrow
question_answer 82) A space ship of mass 2500 kg is to projected so mat it crosses earths gravitational field. If radius of earth is 6400 km. Then initial minimum velocity of space ship must be:
A)
5.6 km/sec
done
clear
B)
7.25 km/sec
done
clear
C)
8.5 km/sec
done
clear
D)
11.2 km/sec
done
clear
View Answer play_arrow
question_answer 83) The values of radius and mass of a planet are half than that of earth then value of g on that planet is :
A)
\[4.9\,m/{{s}^{2}}\]
done
clear
B)
\[9.8\,m/{{s}^{2}}\]
done
clear
C)
\[19.6\,m/{{s}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 84) Surface tension force on water rised in capillary tube is balanced by force \[72\times {{10}^{-6}}N\] acting in downward direction due to weight of water. If surface tension of water is 0.06 N/m. Then internal circumference of capillary tube is:
A)
0.5 mm
done
clear
B)
1 mm
done
clear
C)
1.2 mm
done
clear
D)
1.75 mm
done
clear
View Answer play_arrow
question_answer 85) Critical temperature in terms of van der Waals constants a and b is:
A)
\[\frac{8a}{27Rb}\]
done
clear
B)
\[\frac{27ab}{8R}\]
done
clear
C)
\[\frac{8Rb}{27a}\]
done
clear
D)
\[\frac{27a}{8Rb}\]
done
clear
View Answer play_arrow
question_answer 86) \[{{O}_{2}}\] and \[{{H}_{2}}\] gas are at same temperature \[7{}^\circ K\]. Then average kinetic energy of \[{{O}_{2}}\] molecules in comparision of average kinetic energy of \[{{H}_{2}}\] molecules is:
A)
16 times
done
clear
B)
8 times
done
clear
C)
equal
done
clear
D)
\[\frac{1}{16}\]times
done
clear
View Answer play_arrow
question_answer 87) In an electric dipole value of charge is \[3.2\times {{10}^{-19}}\] coulomb and distance between charges is \[2.4\,\overset{\text{o}}{\mathop{\text{A}}}\,\] It is placed in electric field of \[4\times {{10}^{5}}\,volt/m,\] then dipole moment of dipole is:
A)
\[9.6\times {{10}^{-5}}\] coulomb-metre
done
clear
B)
\[12.8\times {{10}^{-14}}\] coulomb-metre
done
clear
C)
\[7.68\,\times {{10}^{-29}}\] coulomb-metre
done
clear
D)
\[30\times {{10}^{-24}}\] coulomb-metre
done
clear
View Answer play_arrow
question_answer 88) Nucleus of He is revolving in circular orbit of radius 0.8 m. It completes a circle in 2 seconds, then the magnetic induction at the centre of circle is:
A)
\[{{}_{\text{0}}}\text{ }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{T}\]
done
clear
B)
\[\text{1}\text{.6}{{}_{\text{0}}}\text{ }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{T}\]
done
clear
C)
\[\frac{{{}_{\text{0}}}}{2}\text{ }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{T}\]
done
clear
D)
\[\text{2}{{}_{\text{0}}}\text{ }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-19}}}\text{T}\]
done
clear
View Answer play_arrow
question_answer 89) In potentiometer experiment a battery of e.m.f. 3 volt is balanced at a length of 45 cm and e.m.f. of another battery balance at 60 cm length. Then e.m.f. of the battery is:
A)
3 volt
done
clear
B)
4 volt
done
clear
C)
4.5 volt
done
clear
D)
6 volt
done
clear
View Answer play_arrow
question_answer 90) Side of a square coil is 4 cm. A magnetic field normal to plane of coil is changing at a rate of 0.4 tesla/ sec. If resistance of coil is \[2\times {{10}^{-3}}\,ohm,\] then induced current is:
A)
0.16 A
done
clear
B)
0.32 A
done
clear
C)
3.2 A
done
clear
D)
1.6 A
done
clear
View Answer play_arrow
question_answer 91) In an A.C. circuit inductance L = 0.5 H and capacitance \[C=8\,\mu F.\] For maximum current in circuit, value of angular frequency is:
A)
500 rad/sec
done
clear
B)
250 rad/sec
done
clear
C)
150 rad/sec
done
clear
D)
100 rad/sec
done
clear
View Answer play_arrow
question_answer 92) In an electric motor back e.m.f. is maximum:
A)
at the start of motor
done
clear
B)
at the stop of motor
done
clear
C)
at maximum speed of motor
done
clear
D)
same in all case
done
clear
View Answer play_arrow
question_answer 93) A chock coil of negligible resistance connected to 220 volt A.C. source- The 5 mA current flows through it. The average power consumed by chock coil is:
A)
zero
done
clear
B)
11 watt
done
clear
C)
\[44\times {{10}^{3}}\] watt
done
clear
D)
1.1 watt
done
clear
View Answer play_arrow
question_answer 94) Self-inductance of a chock coil is 10 m Hz. When it is connected with 10 volt D.C. source, then power consumed is 20 watt. And when connected with 10 volt A.C. source, the average power consumed is 10 watt. Frequency of A.C. source is :
A)
50 Hz
done
clear
B)
60 Hz
done
clear
C)
80Hz
done
clear
D)
100 Hz
done
clear
View Answer play_arrow
question_answer 95) On a bulb is marked 60 volt and 10 watt and is to be run with an A.C. source of 100 volt. Then inductance of series coil inquired is:
A)
1.53 H
done
clear
B)
2.15 H
done
clear
C)
3.27 H
done
clear
D)
3.89 H
done
clear
View Answer play_arrow
question_answer 96) In an L-C-R circuit C = 25 \[\mu F,\]L= 0.1 Hand R = 25 \[\Omega \] if an e.m.f. E = 310 sin 314 t volt is connected to circuit, then impedence is:
A)
\[57.32\,\Omega \]
done
clear
B)
\[79.48\,\Omega \]
done
clear
C)
\[89.5\,\Omega \]
done
clear
D)
\[99.1\,\Omega \]
done
clear
View Answer play_arrow
question_answer 97) In above equation what inductance must be added in the circuit so the impedence become minimum :
A)
0, 31 H
done
clear
B)
0.35 H
done
clear
C)
1 H
done
clear
D)
2 H
done
clear
View Answer play_arrow
question_answer 98) In question (96) value of current is:
A)
\[i=5.4\,\sin \,(314t+\phi )\]
done
clear
B)
\[i=3.\,\sin \,(314t+\phi )\]
done
clear
C)
\[i=3.\,1\,\sin \,(314t-\phi )\]
done
clear
D)
\[i=3.\,46\,\sin \,(314t+\phi )\]
done
clear
View Answer play_arrow
question_answer 99) In an A.C. circuit current and voltage are: \[i={{i}_{0}}\,\sin \,(\omega t-\pi /2),e={{e}_{0}}\,\sin \,\omega t\] then average power consumed in the circuit is:
A)
\[\frac{1}{2}\,{{i}_{0}}{{e}_{0}}\,watt\]
done
clear
B)
\[{{i}_{0}}{{e}_{0}}\] watt
done
clear
C)
\[ie\]watt
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 100) The correct circuit for full wave rectifier containing a capacitor C and load resistance \[{{R}_{L}}\] is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101) Electronic configuration or \[Al\] is:
A)
\[2,8,3\]
done
clear
B)
\[2,6,5\]
done
clear
C)
\[2,3,8\]
done
clear
D)
\[2,5,6\]
done
clear
View Answer play_arrow
question_answer 102) Equilibrium constant \[{{K}_{c}}\] for the reaction \[2N{{H}_{3}}{{N}_{2}}+3{{H}_{2}}\]is given by the following:
A)
\[\frac{{{[N{{H}_{3}}]}^{2}}}{[{{N}_{2}}]{{[{{H}_{2}}]}^{3}}}\]
done
clear
B)
\[\frac{[{{N}_{2}}]{{[{{H}_{2}}]}^{3}}}{{{[N{{H}_{3}}]}^{2}}}\]
done
clear
C)
\[\frac{3{{[H]}_{2}}[{{N}_{2}}]}{2[N{{H}_{3}}]}\]
done
clear
D)
\[\frac{2[N{{H}_{3}}]}{{{[H]}_{2}}[{{N}_{2}}]}\]
done
clear
View Answer play_arrow
question_answer 103) Which of the following reduce Tollens reagent?
A)
\[HOOC-COOH\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}OOH\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[HCOOH\]
done
clear
View Answer play_arrow
question_answer 104) In natural petrolium is:
A)
saturated hydrocarbon
done
clear
B)
compound of sulphur
done
clear
C)
ring saturated hydrocarbon
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 105) In any system at equilibrium state if temperature, pressure and concentration is changed then the equilibrium shifts in the direction that tends to neutralize the effect of change. This is known as:
A)
Guldberg Wages law
done
clear
B)
Gaylussacs law
done
clear
C)
Avogadros rule
done
clear
D)
Le-Chateliers principle
done
clear
View Answer play_arrow
question_answer 106) \[X+NaN{{O}_{2}}+HCl\xrightarrow{{}}C{{O}_{2}}+{{N}_{2}}+{{H}_{2}}O\] compound X is:
A)
\[C{{H}_{3}}CON{{H}_{2}}~\]
done
clear
B)
\[C{{H}_{3}}NO\]
done
clear
C)
\[N{{H}_{2}}CON{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 107) The main product of the reaction of \[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\] with north is acid is:
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}ONO\]
done
clear
C)
\[C{{H}_{3}}CN\]
done
clear
D)
\[C{{H}_{3}}-N=0\]
done
clear
View Answer play_arrow
question_answer 108) The shape of \[CC{{l}_{4}}\]is:
A)
triangular planer
done
clear
B)
tetrahedral
done
clear
C)
square planar
done
clear
D)
linear
done
clear
View Answer play_arrow
question_answer 109) Cryolite is added to alumina in the extraction of aluminium:
A)
to remove impurity
done
clear
B)
to dissolve alumina
done
clear
C)
to catalyze
done
clear
D)
to obtain pure \[Al\]
done
clear
View Answer play_arrow
question_answer 110) The aqueous solution of a salt is acidic salt is made up of:
A)
Strong acid and weak base
done
clear
B)
Strong base and weak acid
done
clear
C)
Strong acid and strong base
done
clear
D)
Weak acid and weak base
done
clear
View Answer play_arrow
question_answer 111) In atomic pile the \[{{D}_{2}}O\] act as:
A)
fuel
done
clear
B)
controller
done
clear
C)
moderator
done
clear
D)
coolent
done
clear
View Answer play_arrow
question_answer 112) \[C{{H}_{3}}-O-C{{H}_{3}}\] and \[C{{H}_{3}}C{{H}_{2}}OH\] are:
A)
geometrical isomers
done
clear
B)
positional isomers
done
clear
C)
chain isomers
done
clear
D)
functional isomers
done
clear
View Answer play_arrow
question_answer 113) The I. U. P. A. C. name of \[{{(C{{H}_{3}})}_{2}}CH-CH{{(C{{H}_{3}})}_{2}}\] is:
A)
1, 1, 2, 3-tetra methyl ethane
done
clear
B)
2, 3-dimethyl butane
done
clear
C)
1, 2-dimethyl ethane
done
clear
D)
2, 3, 3-trimethyl butane
done
clear
View Answer play_arrow
question_answer 114) Alkaline \[KMn{{O}_{4}}\]solution is called:
A)
Benedict solution
done
clear
B)
Tollens reagent
done
clear
C)
Baeyers reagent
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 115) Which of the following contain acidic hydrogen?
A)
\[C{{H}_{3}}-C\equiv CH\]
done
clear
B)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 116) Which of the following will be obtained by mixing \[50%\] \[NaOH\] to bauxite?
A)
\[Al{{(OH)}_{3}}\]
done
clear
B)
\[Al\]
done
clear
C)
\[Fe\]
done
clear
D)
\[NaAl{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 117) Deviation from Aufbaus principle is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 118) \[C{{H}_{3}}-C\equiv CH+{{H}_{2}}O\xrightarrow{{{H}^{+}}/H{{g}^{2+}}}X\] compound X is:
A)
\[C{{H}_{3}}\text{ }COC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 119) When acetone react with chloroform and \[KOH\] formed hypnotic drug. Its name is:
A)
chloritons
done
clear
B)
chloropicrin
done
clear
C)
asprin
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 120) Brass is:
A)
an element
done
clear
B)
a metal
done
clear
C)
transition metal
done
clear
D)
an alloy
done
clear
View Answer play_arrow
question_answer 121) \[C{{H}_{3}}\text{ }CHO+C{{H}_{3}}\text{ }Mg\text{ }Br\xrightarrow{{}}\text{ }X\]compound X is:
A)
a secondary alcohol
done
clear
B)
a tertiary alcohol
done
clear
C)
a primary alcohol
done
clear
D)
\[C{{H}_{3}}-C-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 122) The enzyme which converts starch into maltose is:
A)
maltase
done
clear
B)
diastase
done
clear
C)
zymase
done
clear
D)
invertase
done
clear
View Answer play_arrow
question_answer 123) \[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\] and \[C{{H}_{3}}-O-{{C}_{3}}{{H}_{7}}\]show following type of isomerism:
A)
metamerism
done
clear
B)
functional group
done
clear
C)
tautomerism
done
clear
D)
position
done
clear
View Answer play_arrow
question_answer 124) The oxidation number of S in \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] is:
A)
\[+6\]
done
clear
B)
\[-6\]
done
clear
C)
\[+4\]
done
clear
D)
\[+8\]
done
clear
View Answer play_arrow
question_answer 125) The shape of electron cloud is determined by:
A)
Azimuthal quantum number
done
clear
B)
Spin quantum number
done
clear
C)
Principle quantum number
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 126) On addition of \[N{{H}_{4}}OH\]in the equilibrium: \[N{{H}_{4}}ClN{{H}_{4}}^{\oplus }+C{{l}^{\bigcirc -}}\]
A)
more \[N{{H}_{4}}\] is formed
done
clear
B)
dissociation of \[N{{H}_{4}}Cl\]increase
done
clear
C)
more \[C{{l}^{-}}\] is formed
done
clear
D)
dissociation of \[N{{H}_{4}}Cl\]decreases
done
clear
View Answer play_arrow
question_answer 127) For an electron \[n=2\]and \[l=1\] the total magnetic quantum numbers are:
A)
\[0\]
done
clear
B)
\[3\]
done
clear
C)
\[2\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 128) An alkene on ozonolysis gives only one compound. Alkene is:
A)
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 129) If the value of pH of solution is 2, then the concentration of \[({{H}^{+}})\] is:
A)
\[{{10}^{-2}}M\]
done
clear
B)
\[2\times 10M\]
done
clear
C)
\[0.001M\]
done
clear
D)
\[0.0001M\]
done
clear
View Answer play_arrow
question_answer 130) Bond with lowest energy is:
A)
\[\pi \] bond
done
clear
B)
hydrogen bond
done
clear
C)
ionic bond
done
clear
D)
o bond
done
clear
View Answer play_arrow
question_answer 131) Dipositive ion of an element has the electronic configuration \[2,8,10\]. Atomic number of the element is:
A)
\[22\]
done
clear
B)
\[24\]
done
clear
C)
\[25\]
done
clear
D)
\[28\]
done
clear
View Answer play_arrow
question_answer 132) The negative part of the addition reagent adds on that C-atom of unsymmetrical alkene which bears the minimum number of H-atoms. The statement is related to:
A)
Peroxide effect
done
clear
B)
Saytzeffs rule
done
clear
C)
Markownikoffs rule
done
clear
D)
Le-Chateliers principle
done
clear
View Answer play_arrow
question_answer 133) \[C{{H}_{3}}COONa\xrightarrow[\Delta ]{\text{Sodalime}}X+N{{a}_{2}}C{{O}_{3}}\].
A)
\[C{{H}_{4}}\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 134) A atom of \[Mn\] have (atomic no. = 25 and mass no. = 55). The nucleus will have:
A)
25 proton
done
clear
B)
55 proton
done
clear
C)
25 neutron
done
clear
D)
55 neutron
done
clear
View Answer play_arrow
question_answer 135) \[RMgI+C{{H}_{3}}OH\xrightarrow{{}}X,\], compound X is:
A)
\[R-R\]
done
clear
B)
\[R-H\]
done
clear
C)
\[R-OH\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 136) Which of the following will form most stable carbonium ion on dehydration:
A)
\[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,-{{(C{{H}_{3}})}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-CH-C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}\text{ }C{{H}_{2}}\text{ }C{{H}_{2}}\text{ }C{{H}_{2}}\text{ }OH\]
done
clear
D)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 137) From left to right in a period ionization potential:
A)
decreases
done
clear
B)
increases.
done
clear
C)
first increases then decreases
done
clear
D)
first decreases then increases
done
clear
View Answer play_arrow
question_answer 138) Washing soda is:
A)
\[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\]
done
clear
B)
\[{{(NaP{{O}_{3}})}_{6}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[N{{a}_{2}}{{B}_{4}}{{O}_{7}}\text{ }.\text{ 10}{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 139) Lithium and magnesium resemble in properties becauses:
A)
their electronic configuration is same
done
clear
B)
ratio of change of size of their cation is approximately same
done
clear
C)
both have approximately same size
done
clear
D)
both are found together in nature.
done
clear
View Answer play_arrow
question_answer 140) Moving down a group:
A)
electronegativity increases
done
clear
B)
ionization potential increases
done
clear
C)
oxidizing strength increases
done
clear
D)
reducing strength increases
done
clear
View Answer play_arrow
question_answer 141) Which of the following property oftenly increases down a group?
A)
Atomic radius
done
clear
B)
Electron affinity
done
clear
C)
Electronegativity
done
clear
D)
Ionization potential
done
clear
View Answer play_arrow
question_answer 142) Power alcohol is:
A)
\[absolute\text{ }alcohol+C{{H}_{3}}OH\]
done
clear
B)
absolute + petrol + benzene
done
clear
C)
\[absolute\text{ }alcohol+C{{H}_{3}}COOH\]
done
clear
D)
\[absolutealcohol+{{C}_{6}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 143) In the extraction of copper, ore is heated in the presence of air at high temperature. This process is called:
A)
roasting
done
clear
B)
calcination
done
clear
C)
smelting
done
clear
D)
distillation
done
clear
View Answer play_arrow
question_answer 144) Carbonium ion with highest stability is:
A)
\[{{(C{{H}_{3}})}_{3}}\overset{+}{\mathop{C}}\,\]
done
clear
B)
\[\overset{+}{\mathop{C}}\,{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{+}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 145) A white precipitate of silver chloride is formed when sodium chloride solution is added to silver nitrate solution because:
A)
\[AgN{{O}_{3}}\]is insoluble in \[NaCl\]solution
done
clear
B)
\[NaCl\] is insoluble in water
done
clear
C)
\[NaCl\] is an inorganic compound
done
clear
D)
\[C{{l}^{-}}\] ions are present in \[NaCl\] solution
done
clear
View Answer play_arrow
question_answer 146) \[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}+22.4kcal\] the favourable condition for the formation of ammonia is:
A)
high temperature and high pressure
done
clear
B)
low temperature and low pressure
done
clear
C)
high temperature and low pressure
done
clear
D)
low temperature and high pressure
done
clear
View Answer play_arrow
question_answer 147) \[C{{H}_{3}}Cl+2Na+C{{H}_{3}}Cl\xrightarrow{{}}\] \[C{{H}_{3}}-C{{H}_{3}}+2NaCl\] This reaction is:
A)
Kolbes electrolysis
done
clear
B)
Williamsons synthesis
done
clear
C)
Wurtz reaction
done
clear
D)
Sabatier Senderens reaction
done
clear
View Answer play_arrow
question_answer 148) Reaction of \[C{{H}_{3}}C1\]with alcoholic \[KOH\] will give:
A)
\[C{{H}_{4}}\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 149) \[{{C}_{2}}{{H}_{5}}COOAg+C{{l}_{2}}\xrightarrow{\Delta }{{C}_{2}}{{H}_{5}}Cl\] \[+AgCl+C{{O}_{2}}\] This reaction is called:
A)
Wurtz-Fitting reaction
done
clear
B)
Hunsdicker reaction
done
clear
C)
Hell-Volhard Zelensky reaction
done
clear
D)
Hoffmann mustard oil reaction
done
clear
View Answer play_arrow
question_answer 150) Pyrite are concentrated by the following method:
A)
gravity separation
done
clear
B)
froth floatation
done
clear
C)
roasting
done
clear
D)
calcination
done
clear
View Answer play_arrow
question_answer 151) The necessary condition for precipitation is:
A)
solubility product \[<\] ionic product
done
clear
B)
solubility product \[>\] ionic product
done
clear
C)
solubility product \[=\] ionic product
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 152) Which of the following reaction does not occurs?
A)
\[Cu+2AgN{{O}_{3}}\xrightarrow{{}}Cu{{(N{{O}_{3}}\text{)}}_{2}}+2Ag\]
done
clear
B)
\[2KBr+{{I}_{2}}\xrightarrow{{}}2KI+B{{r}_{2}}\]
done
clear
C)
\[Fe+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}FeS{{O}_{4}}+{{H}_{2}}\]
done
clear
D)
\[CuO+{{H}_{2}}\xrightarrow{{}}Cu+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 153) \[HCHO+40%\]strong alkaline solution \[\xrightarrow{\,}\] one of the product is:
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[CH\equiv CH\]
done
clear
View Answer play_arrow
question_answer 154) In which of the following reaction the oxidation and reduction complete?
A)
\[NaBr+HCl\text{ }\xrightarrow{{}}NaCl+\text{ }HBr\]
done
clear
B)
\[HBr+AgN{{O}_{3}}\xrightarrow{{}}AgBr+HN{{O}_{3}}\]
done
clear
C)
\[{{H}_{_{2}}}+B{{r}_{2}}\xrightarrow{{}}2HBr\]
done
clear
D)
\[N{{a}_{2}}O+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 155) Which of the following reaction is not affected by the change of pressure:
A)
\[{{N}_{2}}+{{O}_{2}}2NO\]
done
clear
B)
\[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\]
done
clear
C)
\[2S{{O}_{2}}+{{O}_{2}}2S{{O}_{3}}\]
done
clear
D)
\[PC{{l}_{3}}+C{{l}_{2}}PC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 156) Boiling point of ethyl alcohol is higher than dimethyl ether although the molecular weights of both are same. Its reason is:
A)
ethanol has hydrogen bond
done
clear
B)
methyl groups are attached with oxygen in ether
done
clear
C)
ether is insoluble in water
done
clear
D)
dipole moment of ethanol is comparitively more
done
clear
View Answer play_arrow
question_answer 157) No two electron of an atom can have the same set of four quantum numbers this statement is related to:
A)
Faults rule
done
clear
B)
\[(n+l)\]rule
done
clear
C)
Hunds rule
done
clear
D)
Aufbaus principal
done
clear
View Answer play_arrow
question_answer 158) Which of the following will give both iodoform test and Fehlings test?
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[HCHO\]
done
clear
View Answer play_arrow
question_answer 159) In the froth floatation process for the concentration of ore, the ore particles rise to the surface because ore particle:
A)
are light
done
clear
B)
dont get wet by water
done
clear
C)
are insoluble in water
done
clear
D)
have electric charge
done
clear
View Answer play_arrow
question_answer 160) \[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\xrightarrow{{}}\] \[C{{H}_{3}}COC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}+{{C}_{2}}{{H}_{5}}OH\] This reaction is called:
A)
Williamsons synthesis
done
clear
B)
Esterification
done
clear
C)
Trans esterification
done
clear
D)
Claisen condensation
done
clear
View Answer play_arrow
question_answer 161) Which is an amphoteric oxide?
A)
\[MgO\]
done
clear
B)
\[{{K}_{2}}O\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[CuO\]
done
clear
View Answer play_arrow
question_answer 162) For 2p-orbital:
A)
\[n=2,\]\[l=0\]
done
clear
B)
\[n=2,\]\[l=1\]
done
clear
C)
\[n=1,\]\[l=0\]
done
clear
D)
\[n=1,\]\[l=2\]
done
clear
View Answer play_arrow
question_answer 163) Alkaline hydrolysis of an ester is called:
A)
saponification
done
clear
B)
hydrolysis
done
clear
C)
neutralization
done
clear
D)
hydrogenations
done
clear
View Answer play_arrow
question_answer 164) Most of the alloys are made from the following type of element:
A)
f-block
done
clear
B)
p-block
done
clear
C)
d-block
done
clear
D)
s-block
done
clear
View Answer play_arrow
question_answer 165)
In
A)
\[9\sigma \,\,\,9\pi \]
done
clear
B)
\[5\sigma \,\,\,9\pi \]
done
clear
C)
\[9\sigma \,\,\,7\pi \]
done
clear
D)
\[5\sigma \,\,\,8\pi \]
done
clear
View Answer play_arrow
question_answer 166) Oxytocine is:
A)
a hormone
done
clear
B)
an enzyme
done
clear
C)
an antibody
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 167) Picric acid is:
A)
\[2,4,6\]-trinitro phenol
done
clear
B)
\[2,4\]-dinitro phenol
done
clear
C)
\[2,4,6\]-trinitro benzoic acid
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 168) The compound obtained by the reaction of \[C{{H}_{3}}\text{ }Mg\text{ }Br\]with formaldehyde is:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[HCOOH\]
done
clear
View Answer play_arrow
question_answer 169) The correct sequence of die radius of an atom its cation and anion is:
A)
atom > cation > anion
done
clear
B)
atom > cation = anion
done
clear
C)
atom > cation > anion
done
clear
D)
atom < cation > anion
done
clear
View Answer play_arrow
question_answer 170) Which of the following is insoluble in concentrate sulphuric acid?
A)
Benzene
done
clear
B)
Hexene
done
clear
C)
Ethanol
done
clear
D)
n-hexane
done
clear
View Answer play_arrow
question_answer 171) \[C{{H}_{3}}C{{H}_{2}}I+NaOR\to C{{H}_{3}}C{{H}_{2}}OR+NaI\] This reaction is:
A)
Williamsons continuous etherification
done
clear
B)
Curteus reaction
done
clear
C)
Williamsons synthesis
done
clear
D)
Wurtz reaction
done
clear
View Answer play_arrow
question_answer 172) Conjugate base of \[HC{{O}_{3}}^{-}\] is:
A)
\[C{{O}_{3}}^{2-}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[{{H}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 173) Saturated hydrocarbon gives:
A)
electrophilic substitution
done
clear
B)
free radical substitution
done
clear
C)
electrophilic addition
done
clear
D)
free radical addition
done
clear
View Answer play_arrow
question_answer 174) Ethylene can be prepared by the electrolysis of aqueous solution of:
A)
potassium formate
done
clear
B)
potassium succinate
done
clear
C)
potassium malate
done
clear
D)
potassium turner ate
done
clear
View Answer play_arrow
question_answer 175) \[{{N}_{2}}+{{O}_{2}}2NO,\] \[\Delta H=43.2kcal\] Forward reaction is favoured by:
A)
decrease in temperature
done
clear
B)
decrease in pressure
done
clear
C)
increase in pressure
done
clear
D)
increase in temperature
done
clear
View Answer play_arrow
question_answer 176) The electronic configuration of alkaline earth metal is:
A)
\[n{{s}^{2}}(n-1){{d}^{10}}\]
done
clear
B)
\[n{{s}^{2}}\]
done
clear
C)
\[n{{s}^{1}}\]
done
clear
D)
\[n{{p}^{6}}\]
done
clear
View Answer play_arrow
question_answer 177) \[{{C}_{2}}{{H}_{5}}CHO\] reacts with a base to give 3-hydroxybutanol. This reaction is called:
A)
Aldol condensation
done
clear
B)
Claisens condensation
done
clear
C)
Polymerization
done
clear
D)
Riemer-Tiemann reaction
done
clear
View Answer play_arrow
question_answer 178) Which of the following oxidation number is not zero?
A)
\[{{S}_{8}}\]
done
clear
B)
\[{{P}_{4}}\]
done
clear
C)
\[HCHO\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 179) Absolute alcohol from rectified sprit can be prepared by:
A)
hydrolysis
done
clear
B)
steam distillation
done
clear
C)
azeotropic distillation
done
clear
D)
fractional distillation
done
clear
View Answer play_arrow
question_answer 180) Which of the following have ionic bond, covalent bond and co-ordinate bond?
A)
Water
done
clear
B)
Ammonia
done
clear
C)
Potassium bromide
done
clear
D)
Copper sulphate pentahydrate
done
clear
View Answer play_arrow
question_answer 181)
A)
Deals Alder reaction
done
clear
B)
Finkalstine reaction
done
clear
C)
Gattermann reaction
done
clear
D)
Riemer-Tiemann reaction
done
clear
View Answer play_arrow
question_answer 182) Electropositive character in a period from left to right:
A)
first increases then decreases
done
clear
B)
first decreases then increases
done
clear
C)
decreases
done
clear
D)
increases
done
clear
View Answer play_arrow
question_answer 183) The product of the reaction of 2 mole of chloroform with \[4\text{ }mole\]of \[KOH\]:
A)
formic acid
done
clear
B)
succinic acid
done
clear
C)
potassium formate
done
clear
D)
potassium chloride
done
clear
View Answer play_arrow
question_answer 184) Bond angle and bond length in benzene are:
A)
\[{{120}^{o}}\]and \[1.39\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[{{120}^{o}}\] and \[154\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[{{180}^{o}}\] and \[1.33\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[{{120}^{o}}\] and \[1.34\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 185) A catalyst in a reversible equilibrium:
A)
increases the rate of backward reaction
done
clear
B)
increase the rate of forward reaction
done
clear
C)
increases the rate of forward and backward reaction with equal rate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 186) Element with atomic number 33, the proton and number of atom have:
A)
\[33\]
done
clear
B)
\[3\]
done
clear
C)
\[6\]
done
clear
D)
\[9\]
done
clear
View Answer play_arrow
question_answer 187) High hydrocarbon change into low hydrocarbon, this process is known as:
A)
mining
done
clear
B)
cracking
done
clear
C)
isomerization
done
clear
D)
hydroforming
done
clear
View Answer play_arrow
question_answer 188) Which of the following can act as oxidant as well as reductant?
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[NaOH\]
done
clear
View Answer play_arrow
question_answer 189) Which of the following reagent will distinguish between propene and propyne:
A)
Bromine water
done
clear
B)
\[{{[Ag{{(N{{H}_{3}})}_{2}}]}^{+}}\]
done
clear
C)
Alkaline \[KMn{{O}_{4}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 190) Which of the following is dimagnetic?
A)
\[{{O}_{2}}^{-}\]
done
clear
B)
\[NO\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 191) Which of the following Hoffmann bromide reaction?
A)
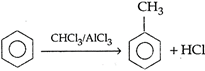
\[C{{H}_{2}}COCl+C{{H}_{3}}OH\xrightarrow{{}}\]\[C{{H}_{2}}COOC{{H}_{3}}HCl\]
done
clear
B)
\[C{{H}_{3}}CON{{H}_{2}}+B{{r}_{2}}+NaOH\xrightarrow{{}}\]\[C{{H}_{3}}N{{H}_{2}}+{{K}_{2}}C{{O}_{3}}+2KBr+2{{H}_{2}}O\]
done
clear
C)
\[RCN+4H\xrightarrow{{}}RCHN{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CN+2{{H}_{2}}\text{O}\xrightarrow{{}}C{{H}_{3}}COOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 192) What is the pH value of \[40ml\text{ }0.10M\,HCl\] solution combind with \[10ml\text{ }0.45M\] \[NaOH\]solution?
A)
\[12\]
done
clear
B)
\[8\]
done
clear
C)
\[10\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 193) Aromatic compound mainly gives:
A)
electrophilic substitution reaction
done
clear
B)
nucleophilic substitution reaction
done
clear
C)
nucleophilic addition reaction
done
clear
D)
electrophilic addition reaction
done
clear
View Answer play_arrow
question_answer 194) Lewis acid is:
A)
\[N{{O}_{2}}^{-}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[C{{l}^{-}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 195) If the concentration of \[Cr{{O}_{4}}^{---}\] in saturated solution of \[AgCr{{O}_{4}},\] then the solubility product is:
A)
\[4\times {{10}^{-8}}\]
done
clear
B)
\[8\times {{10}^{-12}}\]
done
clear
C)
\[16\times {{10}^{-12}}\]
done
clear
D)
\[32\times {{10}^{-12}}\]
done
clear
View Answer play_arrow
question_answer 196)
This reaction is called:
A)
Friedel Craft reaction
done
clear
B)
Schmidt reaction
done
clear
C)
Schotten Baumann reaction
done
clear
D)
Gatter-mann reaction
done
clear
View Answer play_arrow
question_answer 197) \[HCHO\]reacts with \[N{{H}_{3}}\]to give:
A)
formaldehyde
done
clear
B)
formaltne
done
clear
C)
hexamethylene
done
clear
D)
formaldehyde ammonia
done
clear
View Answer play_arrow
question_answer 198) Malachite is the ore of:
A)
\[Ag\]
done
clear
B)
\[Al\]
done
clear
C)
\[Fe\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 199) The following property is not shown by d-block elements:
A)
diamagnetism
done
clear
B)
paramagnetism
done
clear
C)
tendency to form complex compounds
done
clear
D)
variable valency
done
clear
View Answer play_arrow
question_answer 200) If the pH value of solution is 5 then what is the pOH value of solution?
A)
\[8\]
done
clear
B)
\[20\]
done
clear
C)
\[9\]
done
clear
D)
\[21\]
done
clear
View Answer play_arrow
question_answer 201) Metamorphosis in Cockroach is:
A)
complete
done
clear
B)
incomplete
done
clear
C)
holometabolous
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 202) Which animal is extinct from India?
A)
Lion
done
clear
B)
Tiger
done
clear
C)
Dodo
done
clear
D)
Cheetah
done
clear
View Answer play_arrow
question_answer 203) Maximum BMR is present in:
A)
Frog
done
clear
B)
Elephant
done
clear
C)
Horse
done
clear
D)
Rat
done
clear
View Answer play_arrow
question_answer 204) In following layers, which layer is not penetrated by blood cells?
A)
Dermis
done
clear
B)
External epidermis
done
clear
C)
Muscles
done
clear
D)
Hypodermal layer
done
clear
View Answer play_arrow
question_answer 205) Which organ, converts urea into ammonia is?
A)
Kidney
done
clear
B)
Testis
done
clear
C)
Liver
done
clear
D)
Intestine
done
clear
View Answer play_arrow
question_answer 206) How many pairs of cranial nerves are present in Rabbit?
A)
8 pairs
done
clear
B)
10 pairs
done
clear
C)
12 pairs
done
clear
D)
14 pairs
done
clear
View Answer play_arrow
question_answer 207) Sensory organs of Ascaris are:
A)
papillae
done
clear
B)
phasmid
done
clear
C)
amphids
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 208) In which mitosis does not takes place?
A)
Muscle cell
done
clear
B)
Bone cell
done
clear
C)
Epithelial cell
done
clear
D)
Nerve cell
done
clear
View Answer play_arrow
question_answer 209) Pseudopodia is characteristic of following class:
A)
Ciliata
done
clear
B)
Sporozoa
done
clear
C)
Sarcodina
done
clear
D)
Mestigofora
done
clear
View Answer play_arrow
question_answer 210) Cleavage occurs in:
A)
egg
done
clear
B)
zygote
done
clear
C)
nerve cell
done
clear
D)
haploid cell
done
clear
View Answer play_arrow
question_answer 211) Excretory organs of Earthworm are:
A)
body wall
done
clear
B)
flame cells
done
clear
C)
nephridia
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 212) If Hydra is divided into many pieces, then:
A)
each piece convert into complete Hydra
done
clear
B)
only few pieces convert into complete Hydra
done
clear
C)
Hydra will be dead
done
clear
D)
Hydra will reproduce very fast
done
clear
View Answer play_arrow
question_answer 213) Male Cockroach is differ from female Cockroach because in this present:
A)
anal cirrae
done
clear
B)
anal styles
done
clear
C)
antennae
done
clear
D)
mandible
done
clear
View Answer play_arrow
question_answer 214) Who controls the concentration of calcium?
A)
Hypothalamus
done
clear
B)
Thyroid
done
clear
C)
Pituitary gland
done
clear
D)
Thyroid and parathyroid
done
clear
View Answer play_arrow
question_answer 215) A cell in take external substances through:
A)
phagocytesis
done
clear
B)
pinocytosis
done
clear
C)
endocytosis
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 216) Function of golgi body is:
A)
synthesis of protein
done
clear
B)
synthesis of lipid
done
clear
C)
synthesis of carbohydrate
done
clear
D)
oxidative phosphorylation
done
clear
View Answer play_arrow
question_answer 217) Which is not carbohydrate?
A)
Cellulose
done
clear
B)
Glucose
done
clear
C)
Starch
done
clear
D)
Wax
done
clear
View Answer play_arrow
question_answer 218) Formation of glycogen from glucose is called:
A)
glycolysis
done
clear
B)
Krebs cycle
done
clear
C)
citric acid cycle
done
clear
D)
glycogcnesis
done
clear
View Answer play_arrow
question_answer 219) Nerve cells are present in:
A)
Amoeba
done
clear
B)
Sycon
done
clear
C)
Paramecium
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 220) Which of the following doesnt spread disease in human?
A)
Entamoeba histolytica
done
clear
B)
Entamoeba gingiualis
done
clear
C)
Entamoeba coli
done
clear
D)
Plasniodiimi ovale
done
clear
View Answer play_arrow
question_answer 221) Number of testis present in Ascaris:
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 222) In which part of Cockroach exoskeleton is in vaginated:
A)
proctodium
done
clear
B)
stomodium
done
clear
C)
mesenteron
done
clear
D)
both a and b
done
clear
View Answer play_arrow
question_answer 223) Which is not present in protein?
A)
Carbon
done
clear
B)
Sulphur
done
clear
C)
Nitrogen
done
clear
D)
Phosphorus
done
clear
View Answer play_arrow
question_answer 224) Following is not present in prokaryocic cell:
A)
smooth ER
done
clear
B)
mitochondria
done
clear
C)
golgi body
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 225) Nucleic acids are made up of:
A)
nucleosides
done
clear
B)
nucleotides
done
clear
C)
sugar
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 226) Maximum quantity of iron present in:
A)
red blood cells
done
clear
B)
white blood cells
done
clear
C)
bone cells
done
clear
D)
muscle cells
done
clear
View Answer play_arrow
question_answer 227) Which organ is power house of cell?
A)
Nucleus
done
clear
B)
Mitochondria
done
clear
C)
Golgi body
done
clear
D)
Lysosome
done
clear
View Answer play_arrow
question_answer 228) Which of the following only one host is present in life cycle?
A)
Entamoeba histolytica
done
clear
B)
Plasmodium
done
clear
C)
Taenia
done
clear
D)
Liver fluke
done
clear
View Answer play_arrow
question_answer 229) By which process, oxidation of pyruvic acid gives \[C{{O}_{2}}\] and \[{{H}_{2}}O\]?
A)
Krebs cycle
done
clear
B)
Tricarboxylic acid cycle
done
clear
C)
Citric acid cycle
done
clear
D)
Glycogenesis
done
clear
View Answer play_arrow
question_answer 230) Living substance of cell is called:
A)
cytoplasm
done
clear
B)
nucleoplasm
done
clear
C)
protoplasm
done
clear
D)
mitochondrial liquid
done
clear
View Answer play_arrow
question_answer 231) Which is not Chordate characteristic?
A)
Notochord
done
clear
B)
Placenta
done
clear
C)
Dorsal tubular nerve chord
done
clear
D)
Phryngeal gill slits
done
clear
View Answer play_arrow
question_answer 232) Red blood corpuscles of Rabbit are :
A)
round and biconvex
done
clear
B)
oval and biconvex
done
clear
C)
round and biconcave
done
clear
D)
oval and biconcave
done
clear
View Answer play_arrow
question_answer 233) Which ions help is blood coagulation?
A)
\[{{K}^{+}}\]
done
clear
B)
\[C{{a}^{++}}\]
done
clear
C)
\[M{{g}^{++}}\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 234) Pancreas secretes;
A)
insulin
done
clear
B)
glucagon
done
clear
C)
digestive enzymes
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 235) Which of the following is absent in heart of Rabbit?
A)
left auricle
done
clear
B)
left ventricle
done
clear
C)
sinus venosus
done
clear
D)
pace maker
done
clear
View Answer play_arrow
question_answer 236) Glochidium larva presents in :
A)
Bivalvia
done
clear
B)
Gastropoda
done
clear
C)
Cephalopoda
done
clear
D)
Arthropoda
done
clear
View Answer play_arrow
question_answer 237) Retrogressive metamorphosis is present in:
A)
Frog
done
clear
B)
Rabbit
done
clear
C)
Rat
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 238) Choanocytes present in animals of following phylum :
A)
Protozoa
done
clear
B)
Porifera
done
clear
C)
Coelenterata
done
clear
D)
Ptenophora
done
clear
View Answer play_arrow
question_answer 239) Non-poisonous snake is :
A)
Python
done
clear
B)
Viper
done
clear
C)
Cobra
done
clear
D)
Krat
done
clear
View Answer play_arrow
question_answer 240) Arteries which carries blood to diaphragm :
A)
cardiac
done
clear
B)
phrenic
done
clear
C)
comary
done
clear
D)
pulmonary
done
clear
View Answer play_arrow
question_answer 241) Insects egg is:
A)
homolecithal
done
clear
B)
mesolecithal
done
clear
C)
centerolecithal
done
clear
D)
microlecithal
done
clear
View Answer play_arrow
question_answer 242) Which hormone is not synthesized in pituitary gland?
A)
Growth hormone
done
clear
B)
FSH
done
clear
C)
ACTH
done
clear
D)
Vassoprcssin
done
clear
View Answer play_arrow
question_answer 243) Structure derived from ectoderm:
A)
liver
done
clear
B)
muscles
done
clear
C)
brain
done
clear
D)
mesenteries
done
clear
View Answer play_arrow
question_answer 244) Contraction in pupil of eye is due to:
A)
dimlight
done
clear
B)
bright light
done
clear
C)
darkness
done
clear
D)
maximum temperature
done
clear
View Answer play_arrow
question_answer 245) In which group, notochord is restricted to only anterior region ?
A)
Hemichordata
done
clear
B)
Urochordata
done
clear
C)
Cephalochordata
done
clear
D)
Chordata
done
clear
View Answer play_arrow
question_answer 246) How many lobes are present in each cerebral hemisphere?
A)
Two
done
clear
B)
Four
done
clear
C)
Six
done
clear
D)
Eight
done
clear
View Answer play_arrow
question_answer 247) Body cavity if formed by in vagination of:
A)
apomere
done
clear
B)
hypomere
done
clear
C)
mcsomere
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 248) First flying animals were :
A)
Mammals
done
clear
B)
Birds
done
clear
C)
Insects
done
clear
D)
Reptile
done
clear
View Answer play_arrow
question_answer 249) Food of tadpole (Frog) is :
A)
aquatic animals
done
clear
B)
aquatic insects
done
clear
C)
eggs of fishes
done
clear
D)
aquatic vegetation
done
clear
View Answer play_arrow
question_answer 250) Cerebral malaria is spread by following :
A)
Plasmodium uivax
done
clear
B)
Plasmodium ouale
done
clear
C)
Plasmodium Falciparum
done
clear
D)
Plasmodium malariae
done
clear
View Answer play_arrow
question_answer 251) In Rabbit, exoskeleton is in the form of :
A)
hair
done
clear
B)
nails
done
clear
C)
hoof
done
clear
D)
both a and b
done
clear
View Answer play_arrow
question_answer 252) Polyp stage is not present in :
A)
Hydrozoa
done
clear
B)
Scyphozoa
done
clear
C)
Anthozoa
done
clear
D)
Calcarea
done
clear
View Answer play_arrow
question_answer 253) Both cerebral hemisphere are joined by transverse septum which is made up of white matter fibres are called :
A)
corpus callosum
done
clear
B)
corpus albicans
done
clear
C)
corpus spongiossum
done
clear
D)
corpus stratum
done
clear
View Answer play_arrow
question_answer 254) Archenter one is covered by :
A)
ectoderm
done
clear
B)
endoderm
done
clear
C)
mesoderm
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 255) Structure formed due to cleavage is:
A)
zygote
done
clear
B)
morula
done
clear
C)
gastrula
done
clear
D)
neurulla
done
clear
View Answer play_arrow
question_answer 256) Function of chloragogen cells of Earthworm is:
A)
digestion
done
clear
B)
locomotion
done
clear
C)
excretion
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 257) Hydrolytic enzymes are present in :
A)
lysosome
done
clear
B)
mesosomes
done
clear
C)
chondriosome
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 258) Which is nut protein ?
A)
Actin
done
clear
B)
Myosine
done
clear
C)
Albumin
done
clear
D)
Haeme
done
clear
View Answer play_arrow
question_answer 259) In which part of Cockroach both exoskeleton and endoskeleton present?
A)
Mead
done
clear
B)
Thorax
done
clear
C)
Abdomen
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 260) Effective organ for intake of food in Earthworm is:
A)
mouth
done
clear
B)
buccal cavity
done
clear
C)
pharynx
done
clear
D)
stomach
done
clear
View Answer play_arrow
question_answer 261) If nucleus of Amoeba is degenerated, what will be the effect?
A)
Divide immediately
done
clear
B)
Dead after some time
done
clear
C)
No change
done
clear
D)
Rate of growth will be fast
done
clear
View Answer play_arrow
question_answer 262) Infant/juvenile stage of Cockroach is called:
A)
maggot
done
clear
B)
nymph
done
clear
C)
zoea
done
clear
D)
grub
done
clear
View Answer play_arrow
question_answer 263) Dysentery disease spread by :
A)
Amoeba
done
clear
B)
Entamoeba histolytica
done
clear
C)
Plasmodium
done
clear
D)
Entamoeba coli
done
clear
View Answer play_arrow
question_answer 264) Anaema disease is due to the lack of :
A)
calcium
done
clear
B)
copper
done
clear
C)
iron
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 265) Protein synthesis takes place in :
A)
golgi body
done
clear
B)
plasma membrane
done
clear
C)
smooth endoplasmic reticulum
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 266) Quantity of nucleic acid present in cell is approximately :
A)
2%
done
clear
B)
5%
done
clear
C)
10%
done
clear
D)
15%
done
clear
View Answer play_arrow
question_answer 267) Pseudopodia formed, when:
A)
interchange of sol-gel state
done
clear
B)
Amoeba comes in contact with cold water
done
clear
C)
Amoeba comes in contact with hot water
done
clear
D)
Amoeba comes in contact with food
done
clear
View Answer play_arrow
question_answer 268) Sperms of Hydra formed from :
A)
interstitial cells
done
clear
B)
epithelial muscular layer
done
clear
C)
nerve cells
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 269) In which part of Earthworm setae are absent ?
A)
in prostomium
done
clear
B)
in prostomium and clitellum
done
clear
C)
clitellum region
done
clear
D)
clitellum/ first and last segment
done
clear
View Answer play_arrow
question_answer 270) Why does the colour of blood is red in Earthworm?
A)
Due to intracellular haemoglobin
done
clear
B)
Due to intercellular haemoglobin
done
clear
C)
Due to red blood corpuscles
done
clear
D)
Due to white blood corpuscles
done
clear
View Answer play_arrow
question_answer 271) Eggs in which the amount of yolk is very minute, are called :
A)
alecithal
done
clear
B)
microlecithal
done
clear
C)
mesolecithal
done
clear
D)
telolecithal
done
clear
View Answer play_arrow
question_answer 272) Food of Hydra is :
A)
aquatic plants
done
clear
B)
aquatic animals
done
clear
C)
algae and insects
done
clear
D)
small fishes
done
clear
View Answer play_arrow
question_answer 273) Excretory substance of Amoeba is:
A)
ammonia
done
clear
B)
urea
done
clear
C)
uric acid
done
clear
D)
guanine
done
clear
View Answer play_arrow
question_answer 274) Which is not covered by external limiting line boundary?
A)
Ribosome
done
clear
B)
Centrosome
done
clear
C)
Lysosome
done
clear
D)
Spindle
done
clear
View Answer play_arrow
question_answer 275) By which process glucose is converted into \[C{{O}_{2}}\] and \[{{H}_{2}}O\]?
A)
Glycolysis
done
clear
B)
Krebs cycle
done
clear
C)
Pentose phosphate shunt
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 276) Calcium is necessary for:
A)
growth of bone and teeth
done
clear
B)
muscle contraction
done
clear
C)
blood coagulation
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 277) Recapitulation theory was established by:
A)
Muller
done
clear
B)
Von Baer
done
clear
C)
Haeckel
done
clear
D)
both a and c
done
clear
View Answer play_arrow
question_answer 278) Which part of Cockroach wings are not present ?
A)
Prothorax
done
clear
B)
Mesothorax
done
clear
C)
Metathorax
done
clear
D)
None of above
done
clear
View Answer play_arrow
question_answer 279) Entamoeba histolytion is different from Amoeba, because it does not possess:
A)
food vacuole
done
clear
B)
contractile vacuole
done
clear
C)
nucleus
done
clear
D)
nuclear membrane
done
clear
View Answer play_arrow
question_answer 280) Neoteny is present in :
A)
Hyla
done
clear
B)
Tadpole
done
clear
C)
Axolotal
done
clear
D)
Salamander
done
clear
View Answer play_arrow
question_answer 281) Prene glands are present in :
A)
Amphibia
done
clear
B)
Reptiles
done
clear
C)
Birds
done
clear
D)
Mammals
done
clear
View Answer play_arrow
question_answer 282) In which endoskeleton is made up of cartilage?
A)
Dipnoi
done
clear
B)
Mollusca
done
clear
C)
Elasmobranchii
done
clear
D)
Bony fishes
done
clear
View Answer play_arrow
question_answer 283) What is present in Cestoda class?
A)
Absence of alimentary canal
done
clear
B)
Metamerically segmented body
done
clear
C)
Suckers and hook present
done
clear
D)
All the above
done
clear
View Answer play_arrow
question_answer 284) DNA is also present in following:
A)
golgi body
done
clear
B)
lysosome
done
clear
C)
mitochondria
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 285) Acrosome of sperm is formed from:
A)
nucleus
done
clear
B)
mitochondria
done
clear
C)
ribosome
done
clear
D)
golgi body
done
clear
View Answer play_arrow
question_answer 286) Respiratory centre for controlling respiration is situated in:
A)
medulla oblongata
done
clear
B)
cerebellum
done
clear
C)
vagus nerve
done
clear
D)
cerebral hemisphere
done
clear
View Answer play_arrow
question_answer 287) Sea horse is an example of:
A)
Mammalia class
done
clear
B)
Aves class
done
clear
C)
Pisces class
done
clear
D)
Reptile class
done
clear
View Answer play_arrow
question_answer 288) Which animal is not insect?
A)
Ticks
done
clear
B)
Honey bee
done
clear
C)
Beetal
done
clear
D)
Wasps
done
clear
View Answer play_arrow
question_answer 289) Archaeopteryx possess the characteristic of following :
A)
Reptiles
done
clear
B)
Birds
done
clear
C)
Amphibia and Reptiles
done
clear
D)
Reptiles and Birds
done
clear
View Answer play_arrow
question_answer 290) In which phylum, cilia are completely absent?
A)
Annelida
done
clear
B)
Mollusca
done
clear
C)
Arthropoda
done
clear
D)
Porifera
done
clear
View Answer play_arrow
question_answer 291) Hypothalamus is controlling centre of:
A)
hunger and thirst
done
clear
B)
temperature
done
clear
C)
sleep
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 292) Only one primary spermatocyte formed :
A)
one sperm
done
clear
B)
two sperms
done
clear
C)
three sperms
done
clear
D)
four sperms
done
clear
View Answer play_arrow
question_answer 293) Which organ absorbs maximum quantity of iodine?
A)
Thyroid
done
clear
B)
Liver
done
clear
C)
Stomach
done
clear
D)
Pancreas
done
clear
View Answer play_arrow
question_answer 294) In grey matter following is present:
A)
cyton, axon, dendrites
done
clear
B)
dendrites
done
clear
C)
axon
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 295) Kangaroo is member of following order:
A)
primate
done
clear
B)
chiroptera
done
clear
C)
marsupelia
done
clear
D)
monotremata
done
clear
View Answer play_arrow
question_answer 296) Hydra is:
A)
triploblastic
done
clear
B)
diploblastic
done
clear
C)
asymmetric
done
clear
D)
bisymmetric
done
clear
View Answer play_arrow
question_answer 297) Non-stripped muscles are present:
A)
in arteries
done
clear
B)
in veins
done
clear
C)
in urinary bladder
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 298) Most useful organ of Rabbit is:
A)
adrenal medulla
done
clear
B)
testis
done
clear
C)
ovary
done
clear
D)
adrenal cortex
done
clear
View Answer play_arrow
question_answer 299) In which animal poison gland is present?
A)
Male Platypus
done
clear
B)
Female Lizard
done
clear
C)
Male Lizard
done
clear
D)
Frog
done
clear
View Answer play_arrow
question_answer 300) Coelom of Ascaris is called:
A)
schizocoelom
done
clear
B)
enterocoelom
done
clear
C)
pseudocoelom
done
clear
D)
acoelom
done
clear
View Answer play_arrow
question_answer 301) The biggest herbarium of India is situated in:
A)
Dehradun
done
clear
B)
Lucknow
done
clear
C)
Delhi
done
clear
D)
Calcutta
done
clear
View Answer play_arrow
question_answer 302) What is the reason for red colour of snow in snowy region?
A)
Chlamydomonas nivalis
done
clear
B)
Chlamydomonas yellowstonensis
done
clear
C)
Chlorella
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 303) Food, habitat and economic importance of plants are studied in:
A)
Genetics
done
clear
B)
Microbiology
done
clear
C)
Phycology
done
clear
D)
Economic botany
done
clear
View Answer play_arrow
question_answer 304) The synthesis of florigen occurs in:
A)
root
done
clear
B)
leaf
done
clear
C)
branch
done
clear
D)
stem
done
clear
View Answer play_arrow
question_answer 305) The functional unit of chlorophyll is:
A)
stroma
done
clear
B)
grana
done
clear
C)
quantosome
done
clear
D)
oxysome
done
clear
View Answer play_arrow
question_answer 306) Chiasmatas appear in which of the following phase?
A)
Zygotene
done
clear
B)
Diplotene
done
clear
C)
Pachytene
done
clear
D)
Diakinesis
done
clear
View Answer play_arrow
question_answer 307) Mutations are:
A)
mostly useful
done
clear
B)
harmful
done
clear
C)
always useful
done
clear
D)
sometimes useful
done
clear
View Answer play_arrow
question_answer 308) Which of the following accept \[C{{O}_{2}}\] in photosynthesis ?
A)
RuDP
done
clear
B)
ATP
done
clear
C)
ADP
done
clear
D)
PGA
done
clear
View Answer play_arrow
question_answer 309) The value of R. Q. in organic acid will be:
A)
one
done
clear
B)
less than one
done
clear
C)
more than one
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 310) The movement of Mimosa pudica is :
A)
seismonastic
done
clear
B)
chemotrophic
done
clear
C)
geotrophic
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 311) Who proposed the chromosomal theory of in heritance?
A)
Bateson
done
clear
B)
Mendel
done
clear
C)
Robert Hook
done
clear
D)
W.S. Sutton and Boveri
done
clear
View Answer play_arrow
question_answer 312) Double helical structure of DNA was proposed by:
A)
Robert Brown
done
clear
B)
Watson and Crick
done
clear
C)
H.G. Khorana
done
clear
D)
Sutton and Boveri
done
clear
View Answer play_arrow
question_answer 313) Ribosome is consist of:
A)
RNA and protein
done
clear
B)
DNA and protein
done
clear
C)
DNA and RNA
done
clear
D)
DNA, RNA and protein
done
clear
View Answer play_arrow
question_answer 314) The reserve food material in fungi is:
A)
starch
done
clear
B)
protein
done
clear
C)
fat
done
clear
D)
glycogen
done
clear
View Answer play_arrow
question_answer 315) Artificial synthesis of gene was first time done by :
A)
H. G. Khorana
done
clear
B)
Hertwig
done
clear
C)
Strassburger
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 316) The main plant body of bryophyta is:
A)
gametophytic
done
clear
B)
sporophytic
done
clear
C)
thallus
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 317) Who proposed the central dogma?
A)
Crick
done
clear
B)
Khorana
done
clear
C)
Nirenberg
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 318) Product of \[C{{O}_{2}}\] reduction is:
A)
pyruvic acid
done
clear
B)
citric acid
done
clear
C)
tri carboxylic acid
done
clear
D)
glucose
done
clear
View Answer play_arrow
question_answer 319) Embryo formation, without fertilization is called:
A)
apogamy
done
clear
B)
parthenogenesis
done
clear
C)
apomixis
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 320) Cotton fibre is made up if:
A)
cellulose
done
clear
B)
protein
done
clear
C)
fat
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 321) Calotropis and Capparis ecologically grouped under:
A)
hydrophytes
done
clear
B)
xerophytes
done
clear
C)
mesophytes
done
clear
D)
halophytes
done
clear
View Answer play_arrow
question_answer 322) During \[{{C}_{4}}\] pathway, first \[C{{O}_{2}}\] fixation takes place in chloroplast of:
A)
guard cells
done
clear
B)
mesophyll cells
done
clear
C)
spongy parenchyma
done
clear
D)
bundle sheath cells
done
clear
View Answer play_arrow
question_answer 323) Who proposed the histogen theory?
A)
Schmit
done
clear
B)
Hanstein
done
clear
C)
Nagelli
done
clear
D)
Mankey
done
clear
View Answer play_arrow
question_answer 324) Primary tissuess are formed from:
A)
apical and lateral meristem
done
clear
B)
lateral meristem
done
clear
C)
intercalary and apical meristem
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 325) Division in anthers is:
A)
amitosis
done
clear
B)
meiosis
done
clear
C)
mitosis
done
clear
D)
budding
done
clear
View Answer play_arrow
question_answer 326) Purine bases are:
A)
A and T
done
clear
B)
A and C
done
clear
C)
A and G
done
clear
D)
T and C
done
clear
View Answer play_arrow
question_answer 327) When a well irrigated herb plants stem is cut, then xylem sap comes out due to the:
A)
wall pressure
done
clear
B)
root pressure
done
clear
C)
osmatic pressure
done
clear
D)
endosmosis
done
clear
View Answer play_arrow
question_answer 328) Velamen tissue is the characteristic feature of:
A)
parasitic plants
done
clear
B)
epiphytic plants
done
clear
C)
saprophytic plants
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 329) The plant, which does not follow Mendels law of dominance is:
A)
Mirabilis jalapa
done
clear
B)
Pea
done
clear
C)
Gram
done
clear
D)
Chandni
done
clear
View Answer play_arrow
question_answer 330) Who discovered thylakoid?
A)
Mankey
done
clear
B)
Nagelli
done
clear
C)
Hanstein
done
clear
D)
Schmit
done
clear
View Answer play_arrow
question_answer 331) Chipko movement was started from:
A)
Gwalior-(M.P.)
done
clear
B)
GarhwaI-(Uttranchal)
done
clear
C)
Jodhpur-(Rajasthan)
done
clear
D)
Alwar-(Rajasthan)
done
clear
View Answer play_arrow
question_answer 332) 9 : 3 : 3 : 1 follows the Mendels law of:
A)
unit characters
done
clear
B)
dominance
done
clear
C)
independent assortment
done
clear
D)
purity of gametes
done
clear
View Answer play_arrow
question_answer 333) Cross between \[{{F}_{1}}\] generation and pure recessive parent is called:
A)
test cross
done
clear
B)
back cross
done
clear
C)
monohybrid cross
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 334) Genome is the set of chromosomes which remains:
A)
diploid
done
clear
B)
haploid
done
clear
C)
triploid
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 335) The position of stomata in Vallisneria is:
A)
adaxial or abaxial
done
clear
B)
abaxial or adaxial
done
clear
C)
amphistomatal
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 336) Usually root and stomata are absent in:
A)
hydrophytic plants
done
clear
B)
xerophytic plants
done
clear
C)
mesophytic plants
done
clear
D)
halophytic plants
done
clear
View Answer play_arrow
question_answer 337) Mendels laws were rediscovered in:
A)
1890
done
clear
B)
1900
done
clear
C)
1800
done
clear
D)
1905
done
clear
View Answer play_arrow
question_answer 338) Which of the following are nonrenewable resources?
A)
Minerals and fossils
done
clear
B)
Minerals and water
done
clear
C)
Minerals and soil
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 339) In pond ecosystem, the maximum number is represented by;
A)
producers
done
clear
B)
consumers
done
clear
C)
decomposers
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 340) If decomposers are removed from an ecosystem then which of the following will affected?
A)
Producers
done
clear
B)
Biosphere
done
clear
C)
Bio-geochemical cycle
done
clear
D)
Consumers
done
clear
View Answer play_arrow
question_answer 341) Tuberculosis (T.B.) causing bacterium is:
A)
Bacillus coli
done
clear
B)
Mycobacterium tuberculosis
done
clear
C)
Vibrio cholera
done
clear
D)
E. coli
done
clear
View Answer play_arrow
question_answer 342) Quinine is obtained from which part of Cinchona plant?
A)
Bark
done
clear
B)
Leaf
done
clear
C)
Root
done
clear
D)
Flower
done
clear
View Answer play_arrow
question_answer 343) Commercial cork is obtained from:
A)
Quercus suber
done
clear
B)
Peepal
done
clear
C)
Pinus
done
clear
D)
Mango
done
clear
View Answer play_arrow
question_answer 344) Which of the following medicine is used to reduce the blood pressure?
A)
Papavaerin
done
clear
B)
Reserpine
done
clear
C)
Gugal
done
clear
D)
Gum
done
clear
View Answer play_arrow
question_answer 345) What is the relationship between food web and stability of an ecosystem?
A)
If higher the complexity of web the stability will be high
done
clear
B)
Simpler the web/ higher the stability of system.
done
clear
C)
More the webs, lower stability
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 346) Opium is obtained from:
A)
root
done
clear
B)
stem
done
clear
C)
immature fruit
done
clear
D)
leaf
done
clear
View Answer play_arrow
question_answer 347) The main source of energy in alt organisms is:
A)
water
done
clear
B)
sun light
done
clear
C)
nuclear energy
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 348) Whole inflorescence is edible in:
A)
Cauliflower
done
clear
B)
Tomato
done
clear
C)
Brinjal
done
clear
D)
Ladys finger
done
clear
View Answer play_arrow
question_answer 349) Annual rings formed in:
A)
monocot stem
done
clear
B)
monocot root
done
clear
C)
dicot stem
done
clear
D)
dicot root
done
clear
View Answer play_arrow
question_answer 350) Pulses belongs to family:
A)
Malvaceae
done
clear
B)
Leguminosae
done
clear
C)
Cruciferae
done
clear
D)
Solanaceae
done
clear
View Answer play_arrow
question_answer 351) Genetic code on nucleic acid is represented by:
A)
type of nucleic acid
done
clear
B)
number of nucleic acid
done
clear
C)
linear sequence of nucleic acid
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 352) The relation between DPD, OF and TP is:
A)
\[\text{DPD=OP+TP}\]
done
clear
B)
\[\text{DPD+OP-TP}\]
done
clear
C)
\[\text{DPD=}\frac{\text{OP}}{\text{TP}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 353) Axile placentation is present in family:
A)
Malvaceae and Liliaceae
done
clear
B)
Cruciferae
done
clear
C)
Cruciferae and Malvaceae
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 354) Monothecous anther is the characteristic of family;
A)
Malvaceae
done
clear
B)
Liliaceae
done
clear
C)
Solanaceae
done
clear
D)
Cruciferae
done
clear
View Answer play_arrow
question_answer 355) The floral formula of family Cruciterae is:
A)
\[Ebr\,\oplus \,_{\begin{smallmatrix} O \\ + \end{smallmatrix}}^{\uparrow }\,{{K}_{2+2}}{{C}_{\times 4}}{{A}_{2+4}}{{G}_{(\underline{2})}}\]
done
clear
B)
\[\,\oplus \,_{\begin{smallmatrix} O \\ + \end{smallmatrix}}^{\uparrow }\,{{K}_{4}}{{C}_{4}}{{A}_{6}}{{G}_{(2)}}\]
done
clear
C)
\[\,%\,_{\begin{smallmatrix} O \\ + \end{smallmatrix}}^{{}}\,{{K}_{6}}{{C}_{5}}{{A}_{7}}{{G}_{(2)}}\]
done
clear
D)
\[Ebr\,%\,_{\begin{smallmatrix} O \\ + \end{smallmatrix}}^{{}}\,{{K}_{4+4}}{{C}_{4}}{{A}_{4+4}}\,{{G}_{(\underline{2})}}\]
done
clear
View Answer play_arrow
question_answer 356) Sterile stamens are found in:
A)
Cassia fistula (amaltas)
done
clear
B)
Solanum
done
clear
C)
Raphanus sativus
done
clear
D)
Pisum
done
clear
View Answer play_arrow
question_answer 357) Viviparous reproduction is the characteristic of:
A)
Tinospora
done
clear
B)
Xanthomonas
done
clear
C)
Nitrosomonas
done
clear
D)
Rhizophora
done
clear
View Answer play_arrow
question_answer 358) The rhizoids of Riccia are:
A)
unicellular and unbranched
done
clear
B)
unicellular and branched
done
clear
C)
multicellular and unbranched
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 359) The gametophytc generation is dominant in:
A)
gymnosperm
done
clear
B)
bryophyte
done
clear
C)
angiosperm
done
clear
D)
pteridophyta
done
clear
View Answer play_arrow
question_answer 360) Bicollateral vascular bundles are the characteristic of family:
A)
Malvaceae
done
clear
B)
Liliaceae
done
clear
C)
Cucurbitaceae
done
clear
D)
Leguminoseae
done
clear
View Answer play_arrow
question_answer 361) Water is not required in the fertilization of which of die following ?
A)
Riccia
done
clear
B)
Albugo
done
clear
C)
Pteridium
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 362) The male gamete of fem is:
A)
aflagellate
done
clear
B)
uniflagcllate
done
clear
C)
biflagellate
done
clear
D)
multiflagellate
done
clear
View Answer play_arrow
question_answer 363) Hyphae of Albugo are:
A)
aseptate and multinucleate
done
clear
B)
septate and multinucleate
done
clear
C)
aseptate and uninucleate
done
clear
D)
septate and multinucleate
done
clear
View Answer play_arrow
question_answer 364) Cell organelte covered by unit membrane is:
A)
ribosome
done
clear
B)
lysosome
done
clear
C)
mitochondria
done
clear
D)
chloroplast
done
clear
View Answer play_arrow
question_answer 365) Glycolysis takes place in:
A)
mitochondria
done
clear
B)
cytoplasm
done
clear
C)
chloroplast
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 366) Extra nuclear DNA is found in:
A)
chloroplast
done
clear
B)
mitochondria
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 367) Cohesion theory was proposed by:
A)
Dixon and Jolly
done
clear
B)
Boss
done
clear
C)
Vadelloveshky
done
clear
D)
Muller
done
clear
View Answer play_arrow
question_answer 368) Guttation occurs maximum at which time:
A)
noon
done
clear
B)
morning
done
clear
C)
evening
done
clear
D)
night
done
clear
View Answer play_arrow
question_answer 369) How many nucleus take part in the double fertilization of Capsella?
A)
4
done
clear
B)
5
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 370) Symbiotic relation of an alga and fungus is called:
A)
Lichen
done
clear
B)
Mycorrhiza
done
clear
C)
Cuscuta
done
clear
D)
Orchid
done
clear
View Answer play_arrow
question_answer 371) The father of taxonomy is:
A)
Carl Linnaeus
done
clear
B)
Charles Darwin
done
clear
C)
Bentham and Hooker
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 372) The anthers of Capsella are:
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 373) Photosynthetic lamellae in bacteria are found in:
A)
cytoplasm
done
clear
B)
chromoplast
done
clear
C)
leucoplast
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 374) In Ulothrix, the number of chloroplast per cell is;
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 375) Unit which shows henditary characters is:
A)
neutron
done
clear
B)
cistron
done
clear
C)
operon
done
clear
D)
heptanes
done
clear
View Answer play_arrow
question_answer 376) The largest botanical garden of world is:
A)
Indian botanical garden, Calcutta
done
clear
B)
Royal botanical garden, (Kew) England
done
clear
C)
Paris botanical garden
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 377) Nodulated roots are found in:
A)
Malvaceae
done
clear
B)
Leguminosae
done
clear
C)
Solanaceae
done
clear
D)
Compositae
done
clear
View Answer play_arrow
question_answer 378) Mineral salt from soil enters into the cell in the form of:
A)
salts
done
clear
B)
molecules
done
clear
C)
ions
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 379) Which of the following is not emit \[C{{O}_{2}}\] in the environment?
A)
Nuclear energy
done
clear
B)
Oil
done
clear
C)
Coal
done
clear
D)
Gas
done
clear
View Answer play_arrow
question_answer 380) Which of the following is needed to make soil from sand?
A)
Organic matter
done
clear
B)
Soil air
done
clear
C)
Soil water
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 381) Mendels laws were rediscovered by:
A)
de Vries in Holland
done
clear
B)
Bateson in England
done
clear
C)
Nellson in Sweden
done
clear
D)
Linnaeus in England
done
clear
View Answer play_arrow
question_answer 382) The active nucleus is absent in which of the following cell?
A)
Vessel cell
done
clear
B)
Seive tube
done
clear
C)
Tracheid
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 383) 50% time of total cell division is taken by:
A)
\[{{G}_{1}}^{-}\]phase
done
clear
B)
S-phase
done
clear
C)
\[{{G}_{2}}-\]phase
done
clear
D)
M-phase
done
clear
View Answer play_arrow
question_answer 384) Selective permeability is the characteristic of:
A)
cell wall
done
clear
B)
cell membrane
done
clear
C)
cytoplasm
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 385) Only thallus type plant body is the characteristic of which of the following group?
A)
Algae
done
clear
B)
Pteridophyta
done
clear
C)
Bryophyta
done
clear
D)
Gymnospcrm
done
clear
View Answer play_arrow
question_answer 386) Respiratory enzymes are found in:
A)
mitochondria
done
clear
B)
granum
done
clear
C)
chloroplast
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 387) Oval and eccentric starch granules are present in:
A)
Rice
done
clear
B)
Maize
done
clear
C)
Potato
done
clear
D)
Ground nut
done
clear
View Answer play_arrow
question_answer 388) The antibiotic obtained from Streptomyces griseus is:
A)
streptomycin
done
clear
B)
chloromycin
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 389) MAB is:
A)
man and biosphere
done
clear
B)
man, antibody and bacteria
done
clear
C)
Mayer, Addition, Bisbai
done
clear
D)
Man and biology
done
clear
View Answer play_arrow
question_answer 390) Lichens are the indicator of:
A)
water pollution
done
clear
B)
air pollution
done
clear
C)
soil pollution
done
clear
D)
sound pollution
done
clear
View Answer play_arrow
question_answer 391) The function of cytokinin is:
A)
cell division
done
clear
B)
delayin senescence
done
clear
C)
breaking the dormancy
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 392) \[\text{IAA}\] inhibits the growth of:
A)
root
done
clear
B)
leaf
done
clear
C)
stem
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 393) Lenticels are found in:
A)
monocot root
done
clear
B)
monocot stem
done
clear
C)
old dicot stem
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 394) The father of Botany is:
A)
Linnaeus
done
clear
B)
Theophrastus
done
clear
C)
Bentham
done
clear
D)
Hooker
done
clear
View Answer play_arrow
question_answer 395) Which of the following is male gamete?
A)
Androecium
done
clear
B)
Anther
done
clear
C)
Pollen
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 396) The ovule found in about 80% plants is:
A)
orthotropous
done
clear
B)
anatropous
done
clear
C)
semi-anatropous
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 397) Egg apparatus contains:
A)
two antipodals and single egg cell
done
clear
B)
one antipodal and two egg cells
done
clear
C)
one antipodal and three egg cells
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 398) The shape of vascular bundles in monocot is:
A)
D
done
clear
B)
H
done
clear
C)
Y
done
clear
D)
Z
done
clear
View Answer play_arrow
question_answer 399) Which of the following is absent in gymnosperm?
A)
Companion cell
done
clear
B)
Sieve tube
done
clear
C)
Tracheid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 400) The pyramid which remains up right is:
A)
pyramid of biomass
done
clear
B)
pyramid of number
done
clear
C)
pyramid of energy
done
clear
D)
None of the above
done
clear
View Answer play_arrow
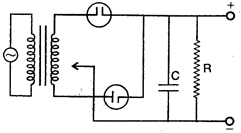
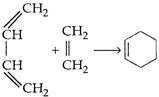 This reaction is called:
This reaction is called: