A) \[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,{{({{C}_{2}}{{H}_{5}})}_{2}}NH,\,\,{{({{C}_{2}}{{H}_{5}})}_{3}}N\]
B) \[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,{{C}_{2}}{{H}_{5}}NH-Cl,\,\,{{C}_{2}}{{H}_{5}}-NC{{l}_{2}}\]
C) \[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,C{{H}_{2}}=C{{H}_{2}},\]\[Cl-C{{H}_{2}}-C{{H}_{2}}-{{C}_{2}}{{H}_{5}}\]
D) \[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,{{({{C}_{2}}{{H}_{5}})}_{3}}N,\,\,{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
Correct Answer: A
Solution :
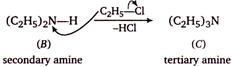
The given reaction is Hermanns ammonolysis method for the formation of amines by alcoholic solution of ammonia and alkyl halides. The Hofmanns ammonolysis of alkyl halides usually gives a mixture of primary, secondary and tertiary amines as illustrated below.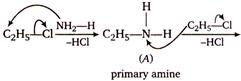
You need to login to perform this action.
You will be redirected in
3 sec
