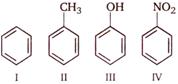
 Correct order of their reactivity in electrophilic substitution reactions would be
Correct order of their reactivity in electrophilic substitution reactions would be
A) \[I>II>III>IV\]
B) \[IV>III>II>I\]
C) \[III>II>I>IV\]
D) \[III>IV>I>II\]
Correct Answer: C
Solution :
In phenol, the lone pair of electrons of \[O-\]atom gets involved in resonance with \[\pi -\]electrons of benzene ring. As a result, the electron density increases in the benzene ring and ring gets activated for electrophilic substitution reaction. Similar effect is shown by \[-C{{H}_{3}}\] group on benzene ring, which does not have a lone pair of electron but increases the electron density on benzene ring by hyper conjugation effect and makes it susceptible for electrophilic substitution. On the other hand, \[-N{{O}_{2}}\] group show electron withdrawing resonance effect \[(-R\]effect) and decreases the electron density in the benzene ring. Therefore the ring gets deactivated and hence further electrophilic substitution becomes difficult. Therefore the order of reactivity in electrophilic substitution reaction should be.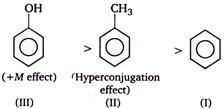
You need to login to perform this action.
You will be redirected in
3 sec
