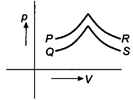
A) P
B) Q
C) R
D) S
Correct Answer: D
Solution :
For \[p-V\] diagrams \[P\] and \[Q\] pressure increases with increase in volume. This is possible when the temperature of the gas increases. Hence, exchange of heat takes place. For \[p-V\] diagrams, \[R\] and \[S\] pressure decreases with increase in volume but the slope of curve is larger for\[S\]. Therefore, it is for an adiabatic process for which no exchange of heat takes place.You need to login to perform this action.
You will be redirected in
3 sec
