A)
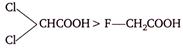 \[>-Cl-C{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH\]
\[>-Cl-C{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH\]
B)
\[F-C{{H}_{2}}COOH>Cl-C{{H}_{2}}COOH\] ![]()
C)
\[HCOOH>C{{H}_{3}}COOH>Cl-C{{H}_{2}}COOH\] ![]()
D) None of the above
Correct Answer: A
Solution :
Group or atom attached with \[-COOH\] group shows positive inductive effect increases electron density on oxygen atom, which suppress the ionization of\[{{H}^{+}}\], hence, acidic strength decreases. Group or atom attached with \[-COOH\] group shows negative inductive effect decreases electron density on oxygen atom, which facilitate the ionization of\[{{H}^{+}}\], hence acidic strength increases. Sequence of group shows negative inductive effect. \[-CHC{{l}_{2}}>C{{H}_{2}}F>-C{{H}_{2}}Cl\] \[-C{{H}_{3}}\]group shows positive inductive effect and \[-H\] doesnt show any effect. So,You need to login to perform this action.
You will be redirected in
3 sec
