A) \[+6\]
B) \[+8\]
C) \[+21\]
D) \[+7\]
Correct Answer: A
Solution :
(i) Draw the structure of oxyacid \[({{H}_{2}}{{S}_{2}}{{O}_{8}})\] to decide the oxidation state of oxygen. Peroxide linkage has \[-1\] oxidation state and normally oxygen has \[-2\] oxidation state.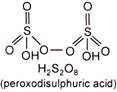 \[\therefore \]Two oxygens form peroxide linkage. \[\therefore \]Oxidation state of two oxygens is \[-1\] each and rest of the six oxygens have \[-2\] oxidation state each, \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] \[+1\times 2+2x+\overset{{{H}_{2}}{{S}_{2}}{{O}_{8}}}{\mathop{(-1\times 2)}}\,+(-2\times 6)=0\] or \[2+2x-2-12=0\] or \[2x=12\] \[\therefore \] \[x=+6\]
\[\therefore \]Two oxygens form peroxide linkage. \[\therefore \]Oxidation state of two oxygens is \[-1\] each and rest of the six oxygens have \[-2\] oxidation state each, \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] \[+1\times 2+2x+\overset{{{H}_{2}}{{S}_{2}}{{O}_{8}}}{\mathop{(-1\times 2)}}\,+(-2\times 6)=0\] or \[2+2x-2-12=0\] or \[2x=12\] \[\therefore \] \[x=+6\]
You need to login to perform this action.
You will be redirected in
3 sec
