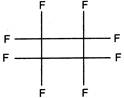
question_answer 1) The unit of surface tension is
A)
N/C
done
clear
B)
N/m
done
clear
C)
C/N
done
clear
D)
m/N
done
clear
View Answer play_arrow
question_answer 2) The unit of Stefan constant is
A)
\[W/{{(K)}^{4}}\]
done
clear
B)
\[eV/{{(K)}^{4}}\]
done
clear
C)
\[W/{{(m)}^{2}}{{(K)}^{4}}\]
done
clear
D)
\[eV/{{(m)}^{2}}{{(K)}^{4}}\]
done
clear
View Answer play_arrow
question_answer 3)
A ball is projected on the strain from the horizontal with velocity u. The maximum number of strains that can be completed crossed is
A)
\[\frac{2{{u}^{2}}h}{g{{b}^{2}}}\]
done
clear
B)
\[\frac{{{u}^{2}}h}{g{{b}^{2}}}\]
done
clear
C)
\[\frac{2{{u}^{2}}b}{gh}\]
done
clear
D)
\[\frac{{{u}^{2}}b}{gh}\]
done
clear
View Answer play_arrow
question_answer 4) The acceleration a of moving body from rest is 2 (t - 1). Then, the velocity at 5 s will be
A)
15 m/s
done
clear
B)
25 m/s
done
clear
C)
35 m/s
done
clear
D)
45 m/s
done
clear
View Answer play_arrow
question_answer 5) The positive charged ball is suspended by thread of silk. If we place a positive charge\[{{q}_{0}}\]at one point and measure the\[F/{{q}_{0}},\]then this can say that electric field E
A)
\[>\frac{F}{{{q}_{0}}}\]
done
clear
B)
\[=\frac{F}{{{q}_{0}}}\]
done
clear
C)
\[=\frac{F}{{{q}_{0}}}\]
done
clear
D)
\[Can't\,be\,measured\]
done
clear
View Answer play_arrow
question_answer 6) If \[Z=\frac{a{{b}^{2}}}{{{c}^{3}}}\], then what is the error in Z? The errors in a, b, c are\[\alpha \],\[\beta \]and \[\gamma \] respectively
A)
\[\alpha \] + 2\[\beta \] - 3\[\gamma \]
done
clear
B)
\[\alpha \]- 2\[\beta \] + 3\[\gamma \]
done
clear
C)
2 \[\alpha \]+ 3\[\beta \] +\[\gamma \]
done
clear
D)
\[\alpha \] + 2\[\beta \]+ 3\[\gamma \]
done
clear
View Answer play_arrow
question_answer 7) The momenta of two particles of masses m and 2m are 2p and p respectively. The ratio of their kinetic energies will be
A)
2 : 1
done
clear
B)
4 : 1
done
clear
C)
1 : 8
done
clear
D)
8 : 1
done
clear
View Answer play_arrow
question_answer 8) If the escape velocity at the earth is 11.2 km/s, then what will be the escape velocity of planet having mass 4 times of earth and gravitational acceleration equal to earth?
A)
7.9 m/s
done
clear
B)
11.2 km/s
done
clear
C)
15.7 km/s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 9) The ratio of radii to the gravitational acceleration of planets X and Y are R and a respectively. The ratio of escape velocities at them, is
A)
\[\sqrt{aR}\]
done
clear
B)
\[\frac{1}{\sqrt{aR}}\]
done
clear
C)
\[aR\]
done
clear
D)
\[\frac{1}{aR}\]
done
clear
View Answer play_arrow
question_answer 10) If the range and time period of projectile are related to\[R=5{{T}^{2}},\]then the angle of projection will be
A)
\[\pi /2\]
done
clear
B)
\[\pi /3\]
done
clear
C)
\[\pi /4\]
done
clear
D)
\[\pi /6\]
done
clear
View Answer play_arrow
question_answer 11) If two masses\[{{m}_{1}}\]and\[{{m}_{2}}\]are dropped freely from the heights\[{{h}_{1}}\]and\[{{h}_{2}}\]respectively, then the ratio of times to reach on earth will be
A)
\[\sqrt{\frac{{{h}_{2}}}{{{h}_{1}}}}\]
done
clear
B)
\[\sqrt{\frac{{{h}_{1}}}{{{h}_{2}}}}\]
done
clear
C)
\[\sqrt{\frac{{{m}_{1}}}{{{m}_{2}}}}\]
done
clear
D)
\[\sqrt{\frac{{{m}_{2}}}{{{m}_{1}}}}\]
done
clear
View Answer play_arrow
question_answer 12) A vehicle starting the motion on any curve path, then how much distance will it cover?
A)
\[\frac{{{u}^{2}}}{4\mu g}\]
done
clear
B)
\[\frac{{{u}^{2}}}{3\mu g}\]
done
clear
C)
\[\frac{{{u}^{2}}}{2\mu g}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 13) At which of the following positions, the acceleration of the particle in SUM will be maximum?
A)
At mean position
done
clear
B)
At peak position
done
clear
C)
At half position of amplitude
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 14)
For following arrangement, the value of equivalent force constant is
A)
\[\frac{3}{2}k\]
done
clear
B)
\[\frac{2}{3}k\]
done
clear
C)
\[2k\]
done
clear
D)
\[3k\]
done
clear
View Answer play_arrow
question_answer 15) The amplitude of any damped oscillation becomes half in 1 min. After 3 s, its amplitude becomes\[\frac{1}{x}\] times of initial amplitude, then\[x\]is \[x\]
A)
\[2\times 3\]
done
clear
B)
\[{{2}^{3}}\]
done
clear
C)
\[{{3}^{2}}\]
done
clear
D)
\[3\times {{2}^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) In friction, the relation between coefficient of kinetic friction \[{{\mu }_{k}}\]and coefficient of static friction \[{{\mu }_{s}}\]is
A)
\[{{\mu }_{k}}\ge {{\mu }_{s}}\]
done
clear
B)
\[{{\mu }_{k}}={{\mu }_{s}}\]
done
clear
C)
\[{{\mu }_{k}}\le {{\mu }_{s}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 17) The ratio of translational kinetic energy to the total kinetic energy for rolling motion of solid sphere without sliding will be
A)
\[\frac{2}{7}\]
done
clear
B)
\[\frac{7}{2}\]
done
clear
C)
\[\frac{5}{7}\]
done
clear
D)
\[\frac{7}{5}\]
done
clear
View Answer play_arrow
question_answer 18) The two particles A and B remain at rest position. Both of them due to their attraction forces move towards each other. At any instant the velocity of A is v. At that instant velocity of B is 2v. The velocity of centre- of mass of the system will be
A)
v
done
clear
B)
zero
done
clear
C)
1.5 v
done
clear
D)
3v
done
clear
View Answer play_arrow
question_answer 19) The dimensional formula of latent heat is
A)
[MLT]
done
clear
B)
\[[{{M}^{0}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[{{M}^{2}}{{L}^{2}}2]\]
done
clear
D)
\[[M{{L}^{0}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 20) A hollow cylinder and a solid cylinder having same mass and diameter are rolled at inclined plane, then which will reach first at the bottom?
A)
Both at same time
done
clear
B)
Hollow cylinder
done
clear
C)
Solid cylinder
done
clear
D)
Which has more density
done
clear
View Answer play_arrow
question_answer 21) A ball is dropped towards the earth from height h, then after three jumps what will be value of height?
A)
\[{{e}^{h/3}}\]
done
clear
B)
\[h{{e}^{6}}\]
done
clear
C)
\[he\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 22) In projectile motion, the value of horizontal range is
A)
\[{{u}^{2}}{{\sin }^{2}}\theta /g\]
done
clear
B)
\[\frac{2{{u}^{2}}\sin \theta \cos \theta }{g}\]
done
clear
C)
\[\frac{{{u}^{2}}\sin 2\theta }{2g}\]
done
clear
D)
\[\frac{{{u}^{2}}\cos 2\theta }{g}\]
done
clear
View Answer play_arrow
question_answer 23) A player takes a complete round in path of radius R in 40 s. After 2 min 20 s, its displacement will be
A)
zero
done
clear
B)
2R
done
clear
C)
\[2\pi R\]
done
clear
D)
\[7\pi R\]
done
clear
View Answer play_arrow
question_answer 24)
Mars is revolving around the sun in elliptical orbit. Its velocity is minimum on
A)
D
done
clear
B)
C
done
clear
C)
B
done
clear
D)
A
done
clear
View Answer play_arrow
question_answer 25) If the mass and radius of planet are twice the earth, then time period of second pendulum on planet will be
A)
1 s
done
clear
B)
2 s
done
clear
C)
\[2\sqrt{2\,s}\]
done
clear
D)
\[\frac{1}{2\sqrt{2}}s\]
done
clear
View Answer play_arrow
question_answer 26)
The particle of mass m moves with velocity v0 and strikes with suspended pendulum of same mass as shown in figure. After completely in elastic collision, the greatest height by particle and pendulum is
A)
\[\frac{{{v}_{0}}^{2}}{4g}\]
done
clear
B)
\[\frac{{{v}_{0}}^{2}}{8g}\]
done
clear
C)
\[\frac{{{v}_{0}}^{2}}{g}\]
done
clear
D)
\[\frac{4{{v}_{0}}^{2}}{g}\]
done
clear
View Answer play_arrow
question_answer 27) In gas, the velocity of sound is v = \[\sqrt{\frac{\gamma RT}{M}}\], the dimensions of \[\gamma \] are
A)
\[[{{M}^{0}}{{T}^{0}}{{T}^{0}}]\]
done
clear
B)
\[[ML{{T}^{-2}}]\]
done
clear
C)
\[[ML{{T}^{-2}}]\]
done
clear
D)
\[[L{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 28) The minimum distance of reflected surface from the source for listening the echo of sound is
A)
28 m
done
clear
B)
18 m
done
clear
C)
19 m
done
clear
D)
16.5 m
done
clear
View Answer play_arrow
question_answer 29) Two sound waves of wavelengths 5 m and 6 m formed 30 beats in 3 s. The velocity of sound is
A)
300 m/s
done
clear
B)
310 m/s
done
clear
C)
320 m/s
done
clear
D)
330 m/s
done
clear
View Answer play_arrow
question_answer 30) Two waves are in phase when
A)
amplitude is same
done
clear
B)
wavelength is same
done
clear
C)
amplitude and wavelength are same
done
clear
D)
phase difference remains constant
done
clear
View Answer play_arrow
question_answer 31)
The effective capacity between points x and y is
A)
C
done
clear
B)
2C/3
done
clear
C)
3C/2
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 32) Newton's law of cooling derived by
A)
Stefan's law
done
clear
B)
Kirchhoffs law
done
clear
C)
Wien's displacement law
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 33) Which of the following laws explains the spectrum of black body?
A)
Rayleigh - Jeans's law
done
clear
B)
Planck's law
done
clear
C)
Wien's displacement law
done
clear
D)
Stefan's law
done
clear
View Answer play_arrow
question_answer 34)
The magnetic field at centre P is
A)
\[\frac{{{\mu }_{0}}I}{4r}\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{2r}\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{8r}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 35) The magnetic field at centre of coil having radius r and current I will be
A)
\[\frac{{{\mu }_{0}}I}{4r}\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{2r}\]
done
clear
C)
zero
done
clear
D)
\[\frac{{{\mu }_{0}}I}{r}\]
done
clear
View Answer play_arrow
question_answer 36) The two charges 7 \[\mu \] C and - 5\[\mu \] C are placed at some distance from each other. They experience a force F. If each of them is given and additional charge of - 2\[\mu \]C, the new force of attraction will be
A)
F
done
clear
B)
2F
done
clear
C)
F/2
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 37) \[{{F}_{g}}\] and\[{{F}_{g}}\]represent the gravitational and electric forces between the electrons at distance 10 cm, then the ratio of \[\frac{{{F}_{g}}}{{{F}_{e}}}\] will be
A)
\[{{10}^{42}}\]
done
clear
B)
10
done
clear
C)
1
done
clear
D)
\[{{10}^{-43}}\]
done
clear
View Answer play_arrow
question_answer 38) The stored energy in capacitor will be
A)
\[\frac{1}{2}{{C}^{2}}V\]
done
clear
B)
\[\frac{1}{2}QV\]
done
clear
C)
\[\frac{1}{2}Q{{V}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 39) The capacity of spherical conductor is 0.1 \[\mu \] F, then its radius will be
A)
90 m
done
clear
B)
900 m
done
clear
C)
9000 m
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 40) Burning 1 g coal gives 2 kcal useful energy. For 1 kWh, the quantity of coal needed will be
A)
\[9kg\]
done
clear
B)
\[\frac{4}{3}kg\]
done
clear
C)
\[\frac{3}{7}kg\]
done
clear
D)
\[\frac{3}{14}kg\]
done
clear
View Answer play_arrow
question_answer 41)
The current I in B circuit is
A)
0.4 A
done
clear
B)
\[-0.4\text{ }A\]
done
clear
C)
0.8 A
done
clear
D)
\[-0.08\text{ }A\]
done
clear
View Answer play_arrow
question_answer 42)
The current\[I\] in the following circuit is
A)
8 A
done
clear
B)
16 A
done
clear
C)
12 A
done
clear
D)
24 A
done
clear
View Answer play_arrow
question_answer 43) According to Kirchhoffs law the algebraic sum of currents meeting at a junction is
A)
limited
done
clear
B)
zero
done
clear
C)
infinite
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 44) If a container filled by the ideal gas keeps in the moving car, the temperature of the gas
A)
will be increased
done
clear
B)
will be decreased
done
clear
C)
will remain unchanged
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 45) The number of turns of primary and secondary coils of transformer are 500 and 5000 respectively. The voltage and frequency of primary coil are 800 V and 50 Hz respectively, then the frequency and voltage of secondary coil will be
A)
50 Hz, 8000 V
done
clear
B)
800 Hz, 800 V
done
clear
C)
50 Hz, 80 V
done
clear
D)
50 Hz, 800 V
done
clear
View Answer play_arrow
question_answer 46) If an ideal gas filled in a closed container and the container is open in vacuum, then its temperature will be
A)
increased
done
clear
B)
decreased
done
clear
C)
unchanged
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 47) In apparatus the values of potential V and flowing current I are given this type \[V=2\text{ }cos\omega \] t volt \[I~=2\text{ }sin\,\omega \] t amp Then, in apparatus the power dissipated will be
A)
zero
done
clear
B)
1.2 W
done
clear
C)
4 W
done
clear
D)
2.0 W
done
clear
View Answer play_arrow
question_answer 48) The power dissipated in AC circuit will be
A)
\[\frac{{{V}_{0}}}{\sqrt{2}}\,\frac{{{I}_{0}}}{\sqrt{2}}\,\cos \,\phi \]
done
clear
B)
\[{{V}_{0}}{{I}_{0}}\cos \phi \]
done
clear
C)
\[\frac{{{V}_{0}}{{I}_{0}}}{4}\,\cos \phi \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 49)
In AC circuit, in resonant position, the reading of which voltmeter is zero?
A)
\[{{V}_{1}}\]
done
clear
B)
\[{{V}_{2}}\]
done
clear
C)
\[{{V}_{3}}\]
done
clear
D)
\[{{V}_{4}}\]
done
clear
View Answer play_arrow
question_answer 50) 1 cm 3 water at 1 atm pressure and\[100{}^\circ C\] converts into\[1671\text{ }c{{m}^{3}}\]steam by taking 54 cal heat. The work done (in cal) during this process will be
A)
400
done
clear
B)
40
done
clear
C)
540
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 51)
In the following circuit, the value of charge on capacitor of capacity 5 \[\mu \] F will be
A)
4.5 \[\mu \]C
done
clear
B)
9.0 \[\mu \]C
done
clear
C)
7 \[\mu \]C
done
clear
D)
30 \[\mu \]C
done
clear
View Answer play_arrow
question_answer 52)
Shown in the given diagram, the electric potential energy of the system will be \[\left( k=\frac{1}{4\pi {{\varepsilon }_{0}}} \right)\]
A)
\[\frac{k{{q}^{2}}}{a}[\sqrt{2}-4]\]
done
clear
B)
\[\frac{k{{q}^{2}}}{a}\]
done
clear
C)
\[0\]
done
clear
D)
\[\frac{kq}{a}[\sqrt{2}-4]\]
done
clear
View Answer play_arrow
question_answer 53)
For balanced Wheatstone bridge, the value of resistance R will be
A)
8\[\Omega \]
done
clear
B)
4\[\Omega \]
done
clear
C)
2\[\Omega \]
done
clear
D)
6\[\Omega \]
done
clear
View Answer play_arrow
question_answer 54) In the full-wave rectifier, the output frequency will be
A)
n
done
clear
B)
2n
done
clear
C)
n/2
done
clear
D)
\[{{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 55) The adiabatic coefficient of a gas is 7/5 .This gas will be
A)
He
done
clear
B)
Ar
done
clear
C)
Ne
done
clear
D)
\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 56)
In the circuit shown in figure, neglecting source resistance the voltmeter and ammeter readings will respectively be (V = 240V)
A)
0, 3 A
done
clear
B)
150 V, 3 A
done
clear
C)
150 V, 6 A
done
clear
D)
0, 8 A
done
clear
View Answer play_arrow
question_answer 57)
Find the value of current I in the given circuit
A)
1 A
done
clear
B)
4 A
done
clear
C)
5 A
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 58) In nth orbit, the angular velocity of electrons is proportional to
A)
\[{{n}^{-1}}\]
done
clear
B)
\[{{n}^{-2}}\]
done
clear
C)
\[{{n}^{-3}}\]
done
clear
D)
\[{{n}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 59) Choose the correct relation
A)
\[y=\,\frac{mglL}{\pi {{r}^{2}}l}\]
done
clear
B)
\[y=\,\frac{mgL}{\pi rl}\]
done
clear
C)
\[y=\,\frac{mg{{\pi }^{2}}}{\pi L}\]
done
clear
D)
\[y=\,\frac{mgr}{\pi L}\]
done
clear
View Answer play_arrow
question_answer 60) Choose the right relation.
A)
\[{{t}_{1/2}}=0.693{{t}_{av}}\]
done
clear
B)
\[{{t}_{av}}=0.693{{t}_{1/2}}\]
done
clear
C)
\[{{t}_{1/2}}=0.414{{t}_{av}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 61) The nature of bond in diamond is
A)
ionic
done
clear
B)
covalent
done
clear
C)
van der Waals'
done
clear
D)
metallic
done
clear
View Answer play_arrow
question_answer 62) Which of the following experiments does represent the wave nature of electrons?
A)
Davisson- Germer
done
clear
B)
de-Broglie
done
clear
C)
Rutherford
done
clear
D)
Millikan oil drop
done
clear
View Answer play_arrow
question_answer 63) The velocity of an electron in the second orbit of sodium atom (atomic number = 1, 1) is v. The velocity of an electron in its fifth orbit will be
A)
\[v\]
done
clear
B)
\[\frac{22}{5}v\]
done
clear
C)
\[\frac{3}{2}v\]
done
clear
D)
\[\frac{2}{5}v\]
done
clear
View Answer play_arrow
question_answer 64) Choose the right equation.
A)
\[\frac{h\lambda }{E}=E\]
done
clear
B)
\[h\lambda =\frac{E}{c}\]
done
clear
C)
\[\frac{hc}{E}=\lambda \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 65) The temperature of star is 6060 K and maximum radiation emitted at 4653 A. For another star, the maximum radiation emitted at 4545 A. The temperature of another star will be
A)
300 K
done
clear
B)
6204 K
done
clear
C)
3000 K
done
clear
D)
120 K
done
clear
View Answer play_arrow
question_answer 66) In a double-slit experiment, the slits are separated by a distance d and the screen is at a distance D from the slits. For monochromatic source of wavelength X, the width of emitted fringe will be
A)
\[\beta =\frac{D\lambda }{d}\]
done
clear
B)
\[\beta =\frac{d\lambda }{D}\]
done
clear
C)
\[\beta =\frac{D\lambda }{d}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 67) A body is walking away from a wall towards an observer at a speed of 2 m/s and blows a whistle whose frequency is 680 Hz. The number of beats heard by the observed per second is (velocity of sound in air = 340 m/s)
A)
zero
done
clear
B)
2
done
clear
C)
8
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 68) A light ray incidents at angle of\[60{}^\circ \]in denser medium in such a way so that reflected and refracted rays remain perpendicular to each other. Refractive index of denser medium relative to rarer medium will be
A)
\[\mu =\sqrt{3}\]
done
clear
B)
\[\mu =\frac{1}{\sqrt{3}}\]
done
clear
C)
\[\mu =\sqrt{2}\]
done
clear
D)
\[\mu =\frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 69) For uranium nucleus, the value of nucleon binding energy will be
A)
7.6eV
done
clear
B)
7.6 \[\mu \]eV
done
clear
C)
7.6 MeV
done
clear
D)
7.6 keV
done
clear
View Answer play_arrow
question_answer 70) The kinetic energy of neutron at temperature 300 K will be\[(m=1.67\times {{10}^{-27}}kg)\]
A)
\[6.21\times ~{{10}^{-21}}J\]
done
clear
B)
\[8.2\times {{10}^{-21}}J\]
done
clear
C)
\[9\times {{10}^{-21}}J\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 71) By which of the following the nucleus can't be cleaved?
A)
\[\alpha -\] ray
done
clear
B)
\[\gamma \] -ray
done
clear
C)
\[\beta \]-ray
done
clear
D)
Laser
done
clear
View Answer play_arrow
question_answer 72) Which of the following is not semiconductor?
A)
\[Si\]
done
clear
B)
\[Ge\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[Cds\]
done
clear
View Answer play_arrow
question_answer 73) For good emissive should be
A)
small work function and small melting point
done
clear
B)
small work function and high melting point
done
clear
C)
high work function and high melting point
done
clear
D)
high work function and small melting point
done
clear
View Answer play_arrow
question_answer 74) The retarding potential for having zero photo-electron current
A)
is proportional to the wavelength of incident light
done
clear
B)
increases uniformly with the increase in the wavelength of incident light
done
clear
C)
is proportional to the frequency of incident light
done
clear
D)
increases uniformly with the increase in the frequency of incident light wave
done
clear
View Answer play_arrow
question_answer 75) From molecular theory of gas which of the following quantities at certainly for all gases will be same?
A)
Momentum
done
clear
B)
Kinetic energy
done
clear
C)
Velocity
done
clear
D)
Mass
done
clear
View Answer play_arrow
question_answer 76) At a given temperature the root mean square velocity of which gas is minimum?
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
He
done
clear
View Answer play_arrow
question_answer 77)
The resonant frequency of the following circuit is
A)
9.3 Hz
done
clear
B)
79 Hz
done
clear
C)
930 Hz
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 78) Which of the following instruments depends upon the coil which rotates in the magnetic field?
A)
Electric motor
done
clear
B)
Dynamo
done
clear
C)
Both [a] & [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 79) The principle of dynamo is
A)
electromagnetic induction
done
clear
B)
magnetic induction
done
clear
C)
electric induction
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 80) In a adiabatic process, initial pressure is 2 atm and final volume is half of initial volume, then final pressure will be (\[\gamma \] = 1.3)
A)
\[{{2}^{2.3}}\]atm
done
clear
B)
4 atm
done
clear
C)
6 atm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 81) Mutual conductance of triode is
A)
\[{{\left( \frac{\Delta {{I}_{p}}}{\Delta {{V}_{g}}} \right)}_{{{V}_{P}}\text{=}\,\text{constant}}}\]
done
clear
B)
\[{{\left( \frac{\Delta {{V}_{g}}}{\Delta {{I}_{g}}} \right)}_{{{V}_{g}}\text{=}\,\text{constant}}}\]
done
clear
C)
\[{{\left( \frac{\Delta {{I}_{p}}}{\Delta {{V}_{p}}} \right)}_{{{V}_{g}}\text{=}\,\text{constant}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 82) The potential of illuminate cathode, grid and plate of triode valve are 0, -3 and 80 V respectively. An electron moves with the kinetic energy of 5eV from the surface of cathode. On reaching the grid, the kinetic energy of electron will be
A)
15 eV
done
clear
B)
2 eV
done
clear
C)
85 eV
done
clear
D)
30 eV
done
clear
View Answer play_arrow
question_answer 83) In photo-electric experiment, for incident light of 4000\[\overset{o}{\mathop{\text{A}}}\,\]the stopping potential is 2 V. If the value of incident light is changed to 3000\[\overset{o}{\mathop{\text{A}}}\,\], the stopping potential will be
A)
2 V
done
clear
B)
less than 2 V
done
clear
C)
zero
done
clear
D)
more than 2 V
done
clear
View Answer play_arrow
question_answer 84) In nuclear fusion the order of binding energy is
A)
\[{{10}^{6}}eV\]
done
clear
B)
\[{{10}^{7}}eV\]
done
clear
C)
\[{{10}^{5}}eV\]
done
clear
D)
\[{{10}^{9}}eV\]
done
clear
View Answer play_arrow
question_answer 85) Choose the correct relation.
A)
\[B=\frac{\Delta p}{-\Delta V/V}\]
done
clear
B)
\[B=-\frac{\Delta V/V}{\Delta p}\]
done
clear
C)
\[B=\frac{\Delta p}{\Delta V}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 86) The root mean square velocity and density of ideal gas are 1840 m/s and \[0.8\text{ }kg/{{m}^{3}}\]respectively. Its pressure will be
A)
\[9.02\times {{10}^{5}}N/{{m}^{2}}\]
done
clear
B)
\[0.02\times {{10}^{4}}N/{{m}^{2}}\]
done
clear
C)
\[9.02\times {{10}^{3}}N/{{m}^{2}}\]
done
clear
D)
\[9.02\times {{10}^{6}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 87) If the pressure at half depth of river is\[\frac{2}{3}\]times of pressure of that bottom, then depth of river is
A)
10 m
done
clear
B)
20 m
done
clear
C)
60 m
done
clear
D)
30 m
done
clear
View Answer play_arrow
question_answer 88) Two equal drops of water with a velocity v is falling in air. If both drops are mixed, then velocity will be
A)
\[2v\]
done
clear
B)
\[\sqrt{2v}\]
done
clear
C)
\[{{2}^{2/3}}v\]
done
clear
D)
\[\frac{v}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 89) 800 identical water drops are combined to form a big drop. Then, the ratio of the final surface energy to the initial surface energy of all the drops together is
A)
1 : 10
done
clear
B)
1 : 15
done
clear
C)
1 : 20
done
clear
D)
1 : 25
done
clear
View Answer play_arrow
question_answer 90) Three light bulbs of 100 W, 200 W and 40 W are connected in series with 200 V source. The current will be
A)
maximum in bulb of 100 W
done
clear
B)
maximum in bulb of 200 W
done
clear
C)
maximum in bulb of 40 W
done
clear
D)
equal in all
done
clear
View Answer play_arrow
question_answer 91) A gas mixture consists of 2 moles of oxygen and 4 moles of argan at temperature T. Neglecting all vibrational moles, the total internal energy of the system is
A)
4 RT
done
clear
B)
15 RT
done
clear
C)
9 RT
done
clear
D)
11 RT
done
clear
View Answer play_arrow
question_answer 92) One mole (\[\gamma \]= 5/3) of monoatomic gas mixture and the one mole of diatomic gas (\[\gamma \]=7/5]. For mixtured the value of y will be
A)
1.40
done
clear
B)
1.50
done
clear
C)
1.53
done
clear
D)
3.07
done
clear
View Answer play_arrow
question_answer 93) The weight of a metal piece in air is 46 g. When it is immersed in a liquid of relative density 1.24 at \[27{}^\circ C,\]then its weight becomes 30 g. When its temperature increases to\[42{}^\circ ,\]the weight of metal piece becomes 30.5 g. If the relative density of liquid at\[42{}^\circ C\]is 1.2, the coefficient of linear expansion of metal will be
A)
\[4.3\,\times \,{{10}^{-5}}{{/}^{o}}C\]
done
clear
B)
\[2.3\,\times \,{{10}^{-5}}{{/}^{o}}C\]
done
clear
C)
\[5.3\,\times \,{{10}^{-5}}{{/}^{o}}C\]
done
clear
D)
\[3.4\,\times \,{{10}^{-5}}{{/}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 94)
How many will be the heat generate in resistance of 500\[\Omega \], when the switch comes from position 1 to position 2?
A)
40\[\times \]\[{{10}^{-3}}J\]
done
clear
B)
\[50\times \,{{10}^{-3}}J\]
done
clear
C)
\[60\times \,{{10}^{-3}}J\]
done
clear
D)
\[30\times \,{{10}^{-3}}J\]
done
clear
View Answer play_arrow
question_answer 95) A short circuited coil is placed in magnetic field at any time. Power loss in coil occurs due to generated induced current. If number of turns becomes 4 times and radius of wire becomes half, then the loss of electric power will be
A)
half
done
clear
B)
equal
done
clear
C)
two times
done
clear
D)
four times
done
clear
View Answer play_arrow
question_answer 96)
The magnetic field induction at centre O will be
A)
\[\frac{3{{\mu }_{0}}{{I}_{a}}}{4a}\]
done
clear
B)
\[\frac{{{\mu }_{0}}{{I}_{a}}}{4\pi a}(1+\pi )\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{4\pi a}\]
done
clear
D)
\[\frac{3{{\mu }_{0}}I}{8a}\]
done
clear
View Answer play_arrow
question_answer 97) Let g be the acceleration due to gravity at earth's surface and K be the rotational kinetic energy of the earth. Suppose the earth's radius decreased by 2% keeping all other quantities same, then
A)
g decreases by 2% and K decreases by 4%
done
clear
B)
g decreases by 4% and K increases by 2%
done
clear
C)
g increases by 4% and K increases by 4%
done
clear
D)
g decreases by 4% and K increases by 4%
done
clear
View Answer play_arrow
question_answer 98) The gravitational force between the point masses m and M is F. Now if an another point mass 2m placed in front and touch of m, then force on M due to m and total force on M will be
A)
2F, F
done
clear
B)
F, 2F
done
clear
C)
F, 3F
done
clear
D)
F, F
done
clear
View Answer play_arrow
question_answer 99) Beat is listened by two sound sources of same amplitude and same frequency. Intensity of sound of maximum beat as compare to the intensity of any source will be
A)
equal
done
clear
B)
two times
done
clear
C)
four times
done
clear
D)
eight times
done
clear
View Answer play_arrow
question_answer 100) The equation of wave is \[y=0.5\text{ }sin(101+x),\] This is moving along x-axis. Its velocity is
A)
10 m/s
done
clear
B)
20 m/s
done
clear
C)
5 m/s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 101) In the following reaction the product will be\[C{{H}_{3}}COOH+H-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-O-H\xrightarrow[{}]{MnO}\]Product
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
D)
\[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 102)
A)
caprolactum
done
clear
B)
phenol
done
clear
C)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) In the following reaction the product will be \[C{{H}_{3}}-Mg-Cl+N{{H}_{2}}-Cl\xrightarrow[{}]{{}}\]Product
A)
\[C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-Cl\]
done
clear
D)
\[C{{H}_{3}}-Mg-N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 104)
A)
done
clear
B)
done
clear
C)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 105)
A)
\[Sn/HCl\]
done
clear
B)
\[SnC{{l}_{2}}+HCl\]
done
clear
C)
\[N{{a}_{2}}S\]
done
clear
D)
\[Na-Hg\]
done
clear
View Answer play_arrow
question_answer 106) Which of the following is nucleophilic pair?
A)
\[\overset{\text{O-}}{\mathop{C}}\,\equiv N,R{{S}^{\text{O-}}}\]
done
clear
B)
\[{}_{\bullet }^{\bullet }{{C}^{\text{O-}}},AlC{{l}_{3}}\]
done
clear
C)
\[B{{r}_{2}},ZnC{{l}_{2}}\]
done
clear
D)
\[\overset{\text{O-}}{\mathop{R}}\,\overset{\oplus }{\mathop{M}}\,gX,{{H}^{\oplus }}\]
done
clear
View Answer play_arrow
question_answer 107)
In the following reaction the product will be
A)
done
clear
B)
phenol
done
clear
C)
benzoic acid
done
clear
D)
benzaldehyde
done
clear
View Answer play_arrow
question_answer 108)
What is the name of the following reaction?
A)
Friedel Craft's reaction
done
clear
B)
Wurtz-Fittig reaction
done
clear
C)
Perkin's reaction
done
clear
D)
Frenkland reaction
done
clear
View Answer play_arrow
question_answer 109) \[C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H+HCN\to X\xrightarrow[{}]{{{H}_{2}}S{{O}_{4}}}\]Lactic acid In the reaction X, will be
A)
acetone
done
clear
B)
acetic acid
done
clear
C)
acetaldehyde cyanohydrin
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 110) IUPAC name of following compound will be \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ CHO \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-C{{H}_{3}}\]
A)
2-isobutyl butanal
done
clear
B)
2-ethyl-3-methyl pentanal
done
clear
C)
3-methyl-4-ethyl pentanal
done
clear
D)
2, 3-diethyl butanal
done
clear
View Answer play_arrow
question_answer 111)
In the following reaction the product will be
A)
done
clear
B)
done
clear
C)
Both [a] and [b]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 112) Which of the following has bond dissociation energy 600 k J/mol?
A)
\[N=N\]
done
clear
B)
\[C-H\]
done
clear
C)
\[C=C\]
done
clear
D)
\[H-H\]
done
clear
View Answer play_arrow
question_answer 113) \[LiAl{{H}_{4}}\]is
A)
a reducing agent
done
clear
B)
a oxidising agent
done
clear
C)
a electrophilic agent
done
clear
D)
a nucleophilic agent
done
clear
View Answer play_arrow
question_answer 114)
The reaction mechanism of the following reaction is done by
A)
free radical mechanism
done
clear
B)
nucleophilic addition and attack
done
clear
C)
electrophilic addition
done
clear
D)
nucleophilic substitution
done
clear
View Answer play_arrow
question_answer 115) \[{{K}_{2}}Mn{{O}_{4}}\]reacts with\[{{O}_{3}}\]to form
A)
\[KMn{{O}_{4}}\]
done
clear
B)
\[Mn{{(OH)}_{2}}\]
done
clear
C)
\[Mn{{O}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 116) Which of the following shows optical isomerism?
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ COOH \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-H\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-COOH\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ COOH \end{smallmatrix}}{\overset{\begin{smallmatrix} COOH \\ | \end{smallmatrix}}{\mathop{C}}}\,-H\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ COOH \end{smallmatrix}}{\overset{\begin{smallmatrix} COOH \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 117) Which of the following is not an ore of magnesium?
A)
Magnasite
done
clear
B)
Dolomite
done
clear
C)
Gypsum
done
clear
D)
Carnalite
done
clear
View Answer play_arrow
question_answer 118) Nitrobenzene on electrolytic reduction with cone.\[{{H}_{2}}S{{O}_{4}}\]gives
A)
p-aminophenol
done
clear
B)
o-aminophenol
done
clear
C)
m-ammophenol
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 119) Bond order in\[{{O}_{2}}\]is
A)
1
done
clear
B)
2
done
clear
C)
1.5
done
clear
D)
2.5
done
clear
View Answer play_arrow
question_answer 120) Electron affinity of nitrogen is
A)
0.21
done
clear
B)
3
done
clear
C)
10.2
done
clear
D)
13.2
done
clear
View Answer play_arrow
question_answer 121) Which of the following test is not given by benzoic acid?
A)
It gives\[C{{O}_{2}}\]with\[NaHC{{O}_{3}}\]
done
clear
B)
Soluble in hot medium and separates on cooling.
done
clear
C)
It gives buffs colour with\[FeC{{l}_{3}}\]solution
done
clear
D)
It gives Libermann's nitroso test
done
clear
View Answer play_arrow
question_answer 122) How much\[{{O}_{2}}\]is required for the complete combustion of\[1\,L\,{{C}_{3}}{{H}_{3}}\]?
A)
0.2 L
done
clear
B)
0.5 L
done
clear
C)
5 L
done
clear
D)
4.8 L
done
clear
View Answer play_arrow
question_answer 123) Which product is obtained, when\[{{C}_{2}}{{H}_{2}}\]passes from red hot copper tube?
A)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
B)
Butadiene
done
clear
C)
\[CH\equiv C-CH=C{{H}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 124)
A)
iodobenzene
done
clear
B)
cyclohexene
done
clear
C)
cyclohexane
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) \[\underset{yellow}{\mathop{MeOH}}\,\underset{red}{\mathop{M{{e}^{\oplus }}}}\,+O{{H}^{\text{O-}}}\] In acidic medium solution will be
A)
red
done
clear
B)
yellow
done
clear
C)
blue
done
clear
D)
colourless
done
clear
View Answer play_arrow
question_answer 126) When a solution passes from a membrane, then the solvent particles pass from the membrane but solute particles does not pass. The membrane is
A)
osmosis membrane
done
clear
B)
diffusion membrane
done
clear
C)
semi-permeable membrane
done
clear
D)
osmotic pressure
done
clear
View Answer play_arrow
question_answer 127) Ethene and ethyne are distinguished by
A)
Baeyer's reagent
done
clear
B)
Tollen's reagent
done
clear
C)
Hager's reagent
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 128) Which of the following shows peroxide effect?
A)
\[HBr\]
done
clear
B)
\[HF\]
done
clear
C)
\[HCl\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 129) Structure of electron cloud of acetylene is
A)
two cylinderical\[\pi -\]cloud
done
clear
B)
unsymmetrical
done
clear
C)
triangular symmetrical
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 130)
The plane in triangular hybridization will be
A)
\[xy\]
done
clear
B)
\[yz\]
done
clear
C)
\[zx\]
done
clear
D)
\[{{x}^{2}}-{{y}^{2}}\]
done
clear
View Answer play_arrow
question_answer 131) Methane + 10%\[{{O}_{2}}\xrightarrow[Cu\,tube]{200{}^\circ C}\]Product; product will be
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}CHO\]
done
clear
View Answer play_arrow
question_answer 132)
A)
Aldol reaction
done
clear
B)
Cannizzaro reaction
done
clear
C)
Schmidt reaction
done
clear
D)
Tischenko reaction
done
clear
View Answer play_arrow
question_answer 133) Acidic hydrolysis of acetoacetic ester gives
A)
acetone
done
clear
B)
acetic acid
done
clear
C)
acetaldehyde
done
clear
D)
methanol
done
clear
View Answer play_arrow
question_answer 134) pH of rain water is
A)
7
done
clear
B)
14
done
clear
C)
0
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 135) When\[[{{H}^{+}}]\]ion concentration is greater than\[{{10}^{-7}}\]then solution will be
A)
acidic
done
clear
B)
basic
done
clear
C)
neutral
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 136) Henderson's equation is
A)
\[pH=p{{K}_{a}}+\log \frac{[salt]}{[acid]}\]
done
clear
B)
\[p{{K}_{a}}=pH-\log \frac{[salt]}{[acid]}\]
done
clear
C)
\[pH=p{{K}_{a}}-\log \frac{[salt]}{[acid]}\]
done
clear
D)
\[p{{K}_{a}}=pH+\log \frac{[salt]}{[acid]}\]
done
clear
View Answer play_arrow
question_answer 137) \[\Delta p\times \Delta x\approx \frac{h}{4\pi }\] This law is given by
A)
Schrodinger
done
clear
B)
Heisenberg's
done
clear
C)
de-Broglie
done
clear
D)
Pauli
done
clear
View Answer play_arrow
question_answer 138)
The correct name of the following freon is
A)
Freon-11
done
clear
B)
Freon-318
done
clear
C)
Freon-12
done
clear
D)
Freon-114
done
clear
View Answer play_arrow
question_answer 139) HF reacts with\[CFC{{l}_{3}}\] in the presence of\[Sb{{F}_{5}}\] to give
A)
Freon-11
done
clear
B)
Freon-12
done
clear
C)
Freon-318
done
clear
D)
Freon-114
done
clear
View Answer play_arrow
question_answer 140) Dilute\[NaCl\]is
A)
basic
done
clear
B)
acidic
done
clear
C)
neutral
done
clear
D)
strong acidic
done
clear
View Answer play_arrow
question_answer 141) Decreasing order of\[-I\]effect is
A)
\[C{{H}_{3}}<C{{H}_{3}}C{{H}_{2}}-<C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-\]
done
clear
B)
\[N{{O}_{2}}<COOH<Cl\]
done
clear
C)
\[Cl>Br>I\]
done
clear
D)
\[Br>Cl>I\]
done
clear
View Answer play_arrow
question_answer 142) pH of\[0.1\text{ }mL\text{ }M\text{ }HCl\]is
A)
1
done
clear
B)
3
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 143) Which of the following shows the minimum ionic character?
A)
\[AgI\]
done
clear
B)
\[MgC{{l}_{2}}\]
done
clear
C)
\[Ba{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
\[HgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 144) \[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow[{}]{Conc.HgS{{O}_{4}}}\Pr oduct\] Product will be
A)
phorone
done
clear
B)
mesitylene
done
clear
C)
paraformaldehyde
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 145) \[CH{{I}_{3}}+Ag\xrightarrow[{}]{{}}Product\] Product be in this reaction
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 146) Given\[{{K}_{a}}=6\times {{10}^{-4}},{{K}_{{{a}_{2}}}}=1.5\times {{10}^{-4}}\]The ratio of relative acidic strength is
A)
4
done
clear
B)
2
done
clear
C)
1
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 147) The second Bohr's radius of hydrogen atom is
A)
\[0.529\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
3.125\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
2.1165\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
4.2\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 148) Which of the following subshell has ten electron?
A)
3d
done
clear
B)
2p
done
clear
C)
4s
done
clear
D)
3p
done
clear
View Answer play_arrow
question_answer 149) In the following reaction \[{{H}_{3}}N+B{{F}_{3}}\xrightarrow[{}]{{}}N{{H}_{3}}\xrightarrow[{}]{{}}B{{F}_{3}}\]
A)
\[B{{F}_{3}}\]is base,\[N{{H}_{3}}\]is acid
done
clear
B)
\[N{{H}_{3}}\]is base,\[B{{F}_{3}}\]is acid
done
clear
C)
Both are acid
done
clear
D)
Both are base
done
clear
View Answer play_arrow
question_answer 150) Which of the following compound has not hydrogen bond?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[HCl\]
done
clear
D)
HF
done
clear
View Answer play_arrow
question_answer 151) Correct order of electron affinity is
A)
\[F>Cl>Br>I\]
done
clear
B)
\[I>Br>Cl>F\]
done
clear
C)
\[Br>I>F>Cl\]
done
clear
D)
\[Cl>F>Br>I\]
done
clear
View Answer play_arrow
question_answer 152) Nylon-66 is made of
A)
hexamethylene diamine
done
clear
B)
thiokol rubber
done
clear
C)
cis-polyisoprene
done
clear
D)
tons-polyisoprene
done
clear
View Answer play_arrow
question_answer 153) Number of conformational isomers of n-butane are
A)
3
done
clear
B)
4
done
clear
C)
6
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 154) When pH increases from 2 to 4 then concentration
A)
increases 100 times
done
clear
B)
decreases 100 times
done
clear
C)
decreases 1000 times
done
clear
D)
increases 1000 times
done
clear
View Answer play_arrow
question_answer 155) Which of the following will give carbylamines reaction?
A)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
D)
\[{{(C{{H}_{3}})}_{4}}{{N}^{\oplus }}\]
done
clear
View Answer play_arrow
question_answer 156) Magnetic moment of\[C{{u}^{+}}\]is
A)
2.59
done
clear
B)
0
done
clear
C)
3.2
done
clear
D)
5.1
done
clear
View Answer play_arrow
question_answer 157) Gangue is removed from its ore by
A)
concentration
done
clear
B)
calcination
done
clear
C)
roasting
done
clear
D)
smelting
done
clear
View Answer play_arrow
question_answer 158) For 2a, 3b, c the Miller indices will be
A)
2, 3, 1
done
clear
B)
1, 2, 4
done
clear
C)
3, 2, 6
done
clear
D)
1, 2, 3
done
clear
View Answer play_arrow
question_answer 159) The wavelength of photon of yellow light is 5890\[\overset{o}{\mathop{\text{A}}}\,\]. Its energy will be
A)
2.3 erg
done
clear
B)
\[3.37\times {{10}^{-12}}erg\]
done
clear
C)
\[4.1\times {{10}^{-8}}erg\]
done
clear
D)
\[1.5\times {{10}^{-2}}erg\]
done
clear
View Answer play_arrow
question_answer 160) Na conducts the electricity because
A)
it releases hydrogen on reaction with water
done
clear
B)
it contains movable electron
done
clear
C)
it consists hydration energy
done
clear
D)
it consists combustion energy
done
clear
View Answer play_arrow
question_answer 161) All d-black elements are
A)
metal
done
clear
B)
non-metal
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 162) Sodium reacts with oxygen to form
A)
monoxide
done
clear
B)
peroxide
done
clear
C)
superoxide
done
clear
D)
\[NaOH\]
done
clear
View Answer play_arrow
question_answer 163) On moving from top to bottom (from\[B{{e}^{2+}}\]to \[B{{e}^{2+}}\]) the degree of hydration
A)
decreases
done
clear
B)
increases
done
clear
C)
first decreases and then increases
done
clear
D)
first increases and then decreases
done
clear
View Answer play_arrow
question_answer 164) From\[B{{e}^{2+}}\]to\[B{{e}^{2+}},\]on moving from top to bottom the ionic radius
A)
decreases
done
clear
B)
increases
done
clear
C)
first decreases and then increases
done
clear
D)
first increases and then decreases
done
clear
View Answer play_arrow
question_answer 165) Haematite is
A)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
B)
\[F{{e}_{3}}{{O}_{4}}\]
done
clear
C)
\[FeC{{O}_{3}}\]
done
clear
D)
\[F{{e}_{2}}{{O}_{3}}.3{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 166) \[{{K}_{2}}C{{r}_{2}}{{O}_{3}}\xrightarrow[{}]{{}}C{{r}^{3+}}\] In the above process, oxidation number of Cr changes to
A)
from 6 to 3
done
clear
B)
from -6 to 3
done
clear
C)
from + 3 to 0
done
clear
D)
from + 6 to 0
done
clear
View Answer play_arrow
question_answer 167) For\[n=1,\text{ }T=1,\text{ }R=\]constant, the relationship between osmotic pressure and volume is
A)
\[p\propto V\]
done
clear
B)
\[p\propto \frac{1}{V}\]
done
clear
C)
\[p=V\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 168) Which of the following has\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},\]\[3{{s}^{2}}3{{p}^{6}},4{{s}^{1}}\]configuration?
A)
K
done
clear
B)
\[Al\]
done
clear
C)
Na
done
clear
D)
\[Ca\]
done
clear
View Answer play_arrow
question_answer 169) Which of the following has maximum ionization energy?
A)
\[(I,{{I}^{+}})\]
done
clear
B)
\[(Br,B{{r}^{+}})\]
done
clear
C)
\[(Li,L{{i}^{+}})\]
done
clear
D)
\[(Cu,C{{u}^{+}})\]
done
clear
View Answer play_arrow
question_answer 170) The reaction is used in bessemerisation process
A)
\[C{{u}_{2}}S+2C{{u}_{2}}O\xrightarrow[{}]{{}}6Cu+S{{O}_{2}}\]
done
clear
B)
\[Si+{{O}_{2}}\xrightarrow[{}]{{}}Si{{O}_{2}}\]
done
clear
C)
\[F{{e}_{2}}{{O}_{3}}+3C\xrightarrow[{}]{{}}2Fe+3CO\]
done
clear
D)
\[4Fe+3{{O}_{2}}\xrightarrow[{}]{{}}2F{{e}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 171) Which of the following relationship is correct for reversible reaction, \[{{H}_{2}}+{{I}_{2}}2HI\]?
A)
\[{{K}_{p}}<1\]
done
clear
B)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
C)
\[{{K}_{p}}>1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 172) For an exothermic reaction, the favourable condition to obtain maximum product is
A)
low pressure
done
clear
B)
low concentration of reactants
done
clear
C)
low temperature
done
clear
D)
high pressure
done
clear
View Answer play_arrow
question_answer 173) When\[CaO\]dissolves in water, then heat releases. On increasing temperature its solubility
A)
increases
done
clear
B)
decreases
done
clear
C)
unchanged
done
clear
D)
first increases, then decreases
done
clear
View Answer play_arrow
question_answer 174) For bcc structure of Na, crystal\[{{N}_{0}}=6\times {{10}^{23}},\]atomic weight of Na =23, radius\[=5\overset{o}{\mathop{\text{A}}}\,,\] then density will be
A)
\[0.6\text{ }g/c{{m}^{3}}\]
done
clear
B)
\[1.2\text{ }g/c{{m}^{3}}\]
done
clear
C)
\[0.4\text{ }g/c{{m}^{3}}\]
done
clear
D)
\[1.0\text{ }g/c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 175) Rate of zero order reaction does not depend on
A)
volume
done
clear
B)
temperature
done
clear
C)
time
done
clear
D)
concentration
done
clear
View Answer play_arrow
question_answer 176) Size of colloidal particle is between
A)
\[1\overset{o}{\mathop{\text{A}}}\,-100\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[10\overset{o}{\mathop{\text{A}}}\,-200\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1000\overset{o}{\mathop{\text{A}}}\,-2000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[>2000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 177) When any 2 g solute dissolves in 50 g water than boiling point comes\[100.5{}^\circ C\]. If\[{{K}_{b}}=0.5\] then molecular weight will be
A)
4
done
clear
B)
40
done
clear
C)
80
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 178) Which of the following is not a characteristic of colloidal particles?
A)
They pass from filter paper easily.
done
clear
B)
They are invisible.
done
clear
C)
They forms heterogeneous mixture.
done
clear
D)
They pass from parchment paper.
done
clear
View Answer play_arrow
question_answer 179) On moving left to right the correct order of hybridization of carbon atoms in compound \[C{{H}_{3}}-CH=C=C{{H}_{2}}.\].
A)
\[s{{p}^{3}},s{{p}^{2}},sp,s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{2}},sp,s{{p}^{2}}\]
done
clear
C)
\[sp,s{{p}^{2}},s{{p}^{2}},s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}},s{{p}^{2}},s{{p}^{2}},sp\]
done
clear
View Answer play_arrow
question_answer 180) In the following reaction X will be \[X+B{{r}^{2}}+KOH\xrightarrow[{}]{{}}C{{H}_{3}}N{{H}_{2}}\]
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-N{{O}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 181) Sodium lauryl sulphate is an example of
A)
neutral detergent
done
clear
B)
cationic detergent
done
clear
C)
anionic detergent
done
clear
D)
soap detergent
done
clear
View Answer play_arrow
question_answer 182) \[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-Cl+KCN\to C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-CN\xrightarrow[{}]{{{H}_{2}}O}X\] Here X will be
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-COOH\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-COOH\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 183)
The correct order of acidic strength of following
I. \[F-C{{H}_{2}}-COOH\]
II.\[Cl-C{{H}_{2}}-COOH\]
III. \[Br-C{{H}_{2}}-COOH\]
A)
\[I>II>III\]
done
clear
B)
\[III>II>I\]
done
clear
C)
\[II>III>I\]
done
clear
D)
\[II>I>III\]
done
clear
View Answer play_arrow
question_answer 184) In the following reaction product will be \[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-ON{{H}_{4}}\xrightarrow[{}]{{{P}_{4}}{{O}_{10}}}Product\]
A)
\[C{{H}_{3}}-C=N\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-C-N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 185) In the following reaction product will be \[nC{{H}_{2}}=CH-O-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow[{}]{Polymerization}Product\]
A)
polyvinyl acetate
done
clear
B)
polyacrylonitrile
done
clear
C)
vinyl acetate
done
clear
D)
acrylonitrile
done
clear
View Answer play_arrow
question_answer 186) Electricity is
A)
mostly positive
done
clear
B)
always negative
done
clear
C)
sometime positive and sometime neutral
done
clear
D)
sometime positive and sometime negative
done
clear
View Answer play_arrow
question_answer 187) Acetic acid is polar in nature but soluble in non-polar solvents because
A)
it forms dimer
done
clear
B)
entropy increases
done
clear
C)
enthalpy decreases
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 188) The ratio of butadiene and styrene in Buna-S polymer is
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[3:1\]
done
clear
D)
\[1:3\]
done
clear
View Answer play_arrow
question_answer 189) An organic compound is purified by electrolytic purification method. It was proposed by
A)
Kolbe
done
clear
B)
Wurtz
done
clear
C)
Frenkland
done
clear
D)
Clemmensen
done
clear
View Answer play_arrow
question_answer 190)
IUPAC name of following compound is
A)
vinyl propene
done
clear
B)
1-isopropene ethylene
done
clear
C)
2-methyl-3-butene
done
clear
D)
3-methyl-1-butene
done
clear
View Answer play_arrow
question_answer 191) Name the compound which obtains sufficient energy for combustion on stirring
A)
methanol
done
clear
B)
chloroform
done
clear
C)
ether
done
clear
D)
power alcohol
done
clear
View Answer play_arrow
question_answer 192) Which of the following smell comes, when some drops of\[{{H}_{2}}S{{O}_{4}}\]adds in acetate solution?
A)
Apple like smell
done
clear
B)
Banana like smell
done
clear
C)
Vinegar like smell
done
clear
D)
White precipitate
done
clear
View Answer play_arrow
question_answer 193) Which of the following is the most stable?
A)
Methane
done
clear
B)
Ethane
done
clear
C)
Butane
done
clear
D)
Propane
done
clear
View Answer play_arrow
question_answer 194) Mixture of camphor and benzoic acid can be separated by
A)
sublimation
done
clear
B)
extraction with a solvent
done
clear
C)
chemical method
done
clear
D)
fractional crystallization
done
clear
View Answer play_arrow
question_answer 195) Which of the following is the example of double salt?
A)
Potash alum
done
clear
B)
Hypo
done
clear
C)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
D)
Bleaching powder
done
clear
View Answer play_arrow
question_answer 196) On passing IF electricity in acid solution, the oxygen liberates
A)
\[11.2\,d{{m}^{3}}\]
done
clear
B)
\[5.6\,d{{m}^{3}}\]
done
clear
C)
\[22.4\text{ }d{{m}^{3}}\]
done
clear
D)
\[1.0\text{ }d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 197) Example of colloidal solution is
A)
alkaline solution of\[{{C}_{6}}{{H}_{5}}COOH\]
done
clear
B)
aqueous solution of\[N{{H}_{2}}CON{{H}_{2}}\]
done
clear
C)
milk
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 198) Which of the following is not an example of molecular crystal?
A)
Hydrogen
done
clear
B)
Iodine
done
clear
C)
Ice
done
clear
D)
Sodium chloride
done
clear
View Answer play_arrow
question_answer 199) Oxidation number of\['N'\] in\[{{N}_{3}}H\]is
A)
\[-\frac{1}{3}\]
done
clear
B)
\[+3\]
done
clear
C)
0
done
clear
D)
\[-3\]
done
clear
View Answer play_arrow
question_answer 200) Chloramphenicol is used for curing in which of the following?
A)
Tuberculosis
done
clear
B)
Typhoid
done
clear
C)
Headache and fever
done
clear
D)
Cough
done
clear
View Answer play_arrow
question_answer 201) Both roots of the following quadratic equation are \[(x-b)(x-c)+(x-c)(x-a)\] \[+(x-a)(x-b)=0\]
A)
positive
done
clear
B)
negative
done
clear
C)
real
done
clear
D)
complex
done
clear
View Answer play_arrow
question_answer 202) For what value of m, the roots of the quadratic equation\[12{{x}^{2}}+mx+5=0\]are in the ratio\[3:2\]?
A)
\[10\sqrt{5}\]
done
clear
B)
\[-5\sqrt{10}\]
done
clear
C)
50
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 203) Maximum value of the expression\[\frac{1}{4{{x}^{2}}+2x+1}\]is
A)
\[\frac{4}{3}\]
done
clear
B)
\[\frac{5}{2}\]
done
clear
C)
\[\frac{13}{4}\]
done
clear
D)
\[\frac{3}{4}\]
done
clear
View Answer play_arrow
question_answer 204) The value of\[\underset{x\to 0}{\mathop{\lim }}\,\frac{\sqrt{\frac{1}{2}(1-\cos 2x)}}{x}\]is
A)
1
done
clear
B)
\[-1\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 205) If the function\[f(x)=\left\{ \begin{matrix} \frac{1-\sin x}{\pi -2x}, & x\ne \frac{\pi }{2} \\ \lambda , & x=\frac{\pi }{2} \\ \end{matrix} \right.\] is continuous at\[x=\frac{\pi }{2},\]then\[\lambda \]is equal to
A)
1
done
clear
B)
\[-1\]
done
clear
C)
\[1/2\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 206) If\[y={{\tan }^{-1}}\left( \frac{\cos x-\sin x}{\cos x+\sin x} \right),\]then\[\frac{dy}{dx}\]is equal to
A)
1
done
clear
B)
\[-1\]
done
clear
C)
\[\sin x\]
done
clear
D)
\[\cos x\]
done
clear
View Answer play_arrow
question_answer 207) Minimum value of\[x+\frac{1}{x}\]is
A)
2
done
clear
B)
\[-2\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 208) If\[x=-1\]and\[x=2\]are the extreme points of the function\[y=a\log |x|+b{{x}^{2}}+x,\]then
A)
\[a=2,b=1/2\]
done
clear
B)
\[a=2,b=-1/2\]
done
clear
C)
\[a=-2,b=-1/2\]
done
clear
D)
\[a=-2,b=1/2\]
done
clear
View Answer play_arrow
question_answer 209) Function\[f(x)=\frac{x}{1+x}-\log (1+x),x>0\]is
A)
decreasing function
done
clear
B)
increasing function
done
clear
C)
not monotonic
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 210) \[\int{{{\cos }^{-1}}\left( \frac{1}{x} \right)}\,dx\]is equal to
A)
\[x{{\sec }^{-1}}x+{{\cosh }^{-1}}x+c\]
done
clear
B)
\[x{{\sec }^{-1}}x-{{\cosh }^{-1}}x+c\]
done
clear
C)
\[{{\cosh }^{-1}}x-{{\sec }^{-1}}x+c\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 211) \[\int{\frac{x{{e}^{x}}}{{{(1+x)}^{2}}}}dx\]is equal to
A)
\[\frac{{{e}^{x}}}{x+1}+c\]
done
clear
B)
\[\frac{{{e}^{x}}}{{{(x+1)}^{2}}}+c\]
done
clear
C)
\[\frac{-{{e}^{x}}}{(x+1)}+c\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 212) \[\int{\cos e{{c}^{4}}xdx}\]is equal to
A)
\[-\cot x+\frac{{{\cot }^{3}}x}{3}+c\]
done
clear
B)
\[\frac{{{\cot }^{3}}x}{3}-\cot x+c\]
done
clear
C)
\[-\cot x-\frac{{{\cot }^{3}}x}{3}+c\]
done
clear
D)
\[\cot x-\frac{{{\cot }^{2}}x}{3}+c\]
done
clear
View Answer play_arrow
question_answer 213) Points\[(-1,1)(0,-3),(5,2)\]and (4, 6) are the vertices of
A)
square
done
clear
B)
parallelogram
done
clear
C)
rectangle
done
clear
D)
rhombus
done
clear
View Answer play_arrow
question_answer 214) Triangle made by the lines\[x+y=0,\] \[3x+y=4,\text{ }x+3y=4\]is
A)
equilateral
done
clear
B)
isosceles
done
clear
C)
right angled
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 215) From any point on the circle\[{{x}^{2}}+{{y}^{2}}={{a}^{2}},\]two tangents are drawn to the circle \[{{x}^{2}}+{{y}^{2}}={{a}^{2}}si{{n}^{2}}\alpha ,\]then angle between them is
A)
\[\frac{\alpha }{2}\]
done
clear
B)
\[\alpha \]
done
clear
C)
\[2\alpha \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 216) A variable chord is drawn to the circle \[{{x}^{2}}+{{y}^{2}}-2ax=0\]from the origin, then the locus of the centre of the circle which is made by taking the chord as the diameter, is
A)
\[{{x}^{2}}+{{y}^{2}}+ax=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}-ax=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}+ay=0\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}-ay=0\]
done
clear
View Answer play_arrow
question_answer 217) The point from which the length of tangents to the circles\[{{x}^{2}}+{{y}^{2}}-8x+40=0,\]\[5{{x}^{2}}+5{{y}^{2}}-25x+80=0\]and \[{{x}^{2}}+{{y}^{2}}-8x+16y+160=0\]are equal, is
A)
\[\left( 8,\frac{15}{2} \right)\]
done
clear
B)
\[\left( -8,\frac{15}{2} \right)\]
done
clear
C)
\[\left( 8,-\frac{15}{2} \right)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 218) The point where the tangent to the curve \[y={{e}^{2x}}\]at (0,1) meets\[x-\]axis, is
A)
(1, 0)
done
clear
B)
\[(-1,0)\]
done
clear
C)
\[\left( -\frac{1}{2},0 \right)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 219) Image of the point\[(4,-3)\]with respect to the line\[y=x,\]is
A)
\[(-4,-3)\]
done
clear
B)
\[(3,-4)\]
done
clear
C)
\[(-3,4)\]
done
clear
D)
\[(-3,-4)\]
done
clear
View Answer play_arrow
question_answer 220) If\[a+b+c=0,\]then the line\[3ax+4by+c=0\]passes through a fixed point which has the coordinate
A)
\[\left( \frac{1}{3},\frac{1}{4} \right)\]
done
clear
B)
\[\left( -\frac{1}{3},\frac{1}{4} \right)\]
done
clear
C)
\[\left( \frac{1}{3},-\frac{1}{4} \right)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 221) The tangents drawn from the focal chord PQ to the parabola meets the directrix at point Q. Then angle PSQ is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{3}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 222) If\[\overrightarrow{A}=2\hat{i}+2\hat{j}+3\hat{k},\overrightarrow{B}=-\hat{i}+2\hat{j}+\hat{k}\]and\[\overrightarrow{C}=3\hat{i}+\hat{j}\]and\[(\overrightarrow{A}+t\overrightarrow{B})\bot \overrightarrow{C},\]ihent is equal to
A)
5
done
clear
B)
4
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 223) If sum of two unit vectors is also a unit vector, then angle between them is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[\frac{2\pi }{3}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
question_answer 224) \[\overrightarrow{a}.\{(\overrightarrow{b}+\overrightarrow{c})\times (\overrightarrow{a}+\overrightarrow{b}+\overrightarrow{c})\}\]is equal to
A)
\[2[\overrightarrow{a}\overrightarrow{b}\overrightarrow{c}]\]
done
clear
B)
0
done
clear
C)
\[[\overrightarrow{a}\overrightarrow{b}\overrightarrow{c}]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 225) Area of small part between the circle\[{{x}^{2}}+{{y}^{2}}=9\]and the line\[x=1\]is
A)
\[{{\sec }^{-1}}3-\sqrt{8}\]
done
clear
B)
\[9{{\sec }^{-1}}3-\sqrt{8}\]
done
clear
C)
\[9\cos e{{c}^{-1}}3-\sqrt{8}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 226) Area between the vertex and the latusrectum of the parabola\[{{y}^{2}}=4ax\]is
A)
\[\frac{4}{3}{{a}^{2}}sq\,units\]
done
clear
B)
\[\frac{8}{3}{{a}^{2}}sq\,units\]
done
clear
C)
\[\frac{2}{3}{{a}^{2}}sq\,units\]
done
clear
D)
\[\frac{5}{3}{{a}^{2}}sq\,units\]
done
clear
View Answer play_arrow
question_answer 227) \[\int_{0}^{\infty }{\log \left( x+\frac{1}{x} \right)}\frac{dx}{1+{{x}^{2}}}\]is equal to
A)
\[-\frac{\pi }{2}\log 2\]
done
clear
B)
\[-\pi \log 2\]
done
clear
C)
\[\pi \log 2\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 228) Intersection point of the tangents drawn to the parabola\[{{y}^{2}}=4ax\]from the points \[(at_{1}^{2},2a{{t}_{1}})\]and\[(at_{2}^{2},2a{{t}_{2}})\]is
A)
\[(a{{t}_{1}}{{t}_{2}},a({{t}_{1}}+{{t}_{2}}))\]
done
clear
B)
\[(a({{t}_{1}}+{{t}_{2}}),a{{t}_{1}}{{t}_{2}})\]
done
clear
C)
\[(t_{1}^{2}t_{2}^{2},{{t}_{1}}+{{t}_{2}})\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 229) If a line makes the angles\[\alpha ,\beta ,\gamma \]and\[\delta \]respectively with all the four diagonals of a cube, then\[co{{s}^{2}}\alpha +co{{s}^{2}}\beta +co{{s}^{2}}\gamma +co{{s}^{2}}\delta \]is equal to
A)
3
done
clear
B)
4
done
clear
C)
4/3
done
clear
D)
3/4
done
clear
View Answer play_arrow
question_answer 230) The cosine of the angle between any two diagonals of a cube is
A)
\[\frac{1}{3}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{2}{3}\]
done
clear
D)
\[\frac{1}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 231) Line\[\frac{x-2}{3}=\frac{y-3}{4}=\frac{z-4}{0}\]is
A)
parallel to yz-plane
done
clear
B)
parallel to zx-plane
done
clear
C)
perpendicular to z-axis
done
clear
D)
parallel to z-axis
done
clear
View Answer play_arrow
question_answer 232) Total number of lines having same slope with the coordinate axes, is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 233) A line makes an angle of\[60{}^\circ \]with\[x\]and y-axes, then the angle which is made by the line with the positive direction of to z-axis, is
A)
\[45{}^\circ \]or \[135{}^\circ \]
done
clear
B)
\[60{}^\circ \]or\[90{}^\circ \]
done
clear
C)
\[90{}^\circ \]or\[120{}^\circ \]
done
clear
D)
\[60{}^\circ \]or\[120{}^\circ \]
done
clear
View Answer play_arrow
question_answer 234) Equation of line passing through the points\[\overrightarrow{a}={{a}_{1}}\hat{i}+{{a}_{2}}\hat{j}+{{a}_{3}}\hat{k}\]and \[\overrightarrow{b}={{b}_{1}}\hat{i}+{{b}_{2}}\hat{j}+{{b}_{3}}\hat{k}\]is (where t is a scalar)
A)
\[\overrightarrow{r}=\overrightarrow{a}+\overrightarrow{b}\]
done
clear
B)
\[\overrightarrow{r}=\overrightarrow{a}+t(\overrightarrow{b}-\overrightarrow{a})\]
done
clear
C)
\[\overrightarrow{r}=\overrightarrow{a}+t\overrightarrow{b}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 235) Polar of the circle\[{{x}^{2}}+{{y}^{2}}=5\]with respect to the point\[(1,-2)\]is a line for the circle \[{{x}^{2}}+{{y}^{2}}-8x+6y+20=0\]which is
A)
a tangent
done
clear
B)
cut at two real points
done
clear
C)
cut at two imaginary points
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 236) The ratio in which the line made by joining the points\[(2,-1,-3)\]and\[(3,2,-1)\]cuts the yz-plane, is
A)
\[-2:3\]
done
clear
B)
\[3:-2\]
done
clear
C)
\[1:2\]
done
clear
D)
\[2:1\]
done
clear
View Answer play_arrow
question_answer 237) If\[|z+4|\le 3,\] then maximum value of\[|z+1|\] is
A)
0
done
clear
B)
4
done
clear
C)
3
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 238) If\[z=x+iy\]and are\[\left( \frac{z-2}{z+2} \right)=\frac{\pi }{6},\]then locus of \[z\]is a
A)
straight line
done
clear
B)
circle
done
clear
C)
parabola
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 239) The value of\[\frac{{{(\cos \theta +i\sin \theta )}^{4}}}{{{(\sin \theta +i\cos \theta )}^{5}}}\]is
A)
\[\cos 9\theta -i\sin 9\theta \]
done
clear
B)
\[\cos \theta -i\sin \theta \]
done
clear
C)
\[\sin 9\theta +i\cos 9\theta \]
done
clear
D)
\[\sin 9\theta -i\cos 9\theta \]
done
clear
View Answer play_arrow
question_answer 240) \[{{(1+i)}^{6}}+{{(1-i)}^{6}}\]is equal to
A)
\[16i\]
done
clear
B)
16
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 241) If\[z=\frac{\sqrt{3}+i}{2},\]then\[{{z}^{69}}\]is equal to
A)
\[1\]
done
clear
B)
2
done
clear
C)
\[-2\]
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 242) \[cosh\text{ }2x\] is equal to
A)
\[cos{{h}^{2}}x-sin{{h}^{2}}x\]
done
clear
B)
\[2\text{ }cos{{h}^{2}}x-1\]
done
clear
C)
\[1-2\text{ }sin{{h}^{2}}x\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 243) If\[x\]is real, then\[cos{{h}^{-1}}x\]is equal to
A)
\[\log (x+\sqrt{{{x}^{2}}+1})\]
done
clear
B)
\[\log (x\pm \sqrt{{{x}^{2}}-1})\]
done
clear
C)
\[\log (x\mp \sqrt{{{x}^{2}}+1})\]
done
clear
D)
\[\log (x-\sqrt{{{x}^{2}}-1})\]
done
clear
View Answer play_arrow
question_answer 244) If \[{{\tan }^{-1}}(\alpha +i\beta )=x+iy,\]then\[x\]is equal to
A)
\[\frac{1}{2}\tan \left( \frac{2\alpha }{1+{{\alpha }^{2}}+{{\beta }^{2}}} \right)\]
done
clear
B)
\[\frac{1}{2}{{\tan }^{-1}}\left( \frac{2\alpha }{1+{{\alpha }^{2}}+{{\beta }^{2}}} \right)\]
done
clear
C)
\[\frac{1}{2}{{\tanh }^{-1}}\left( \frac{2\beta }{1+{{\alpha }^{2}}+{{\beta }^{2}}} \right)\]
done
clear
D)
\[\frac{1}{2}{{\tan }^{-1}}\left( \frac{2\beta }{1-{{\alpha }^{2}}-{{\beta }^{2}}} \right)\]
done
clear
View Answer play_arrow
question_answer 245) Determinant\[\left| \begin{matrix} 1 & a & {{a}^{2}}-bc \\ 1 & b & {{b}^{2}}-ac \\ 1 & c & {{c}^{2}}-ab \\ \end{matrix} \right|\]is equal to
A)
\[a+b+c\]
done
clear
B)
\[{{a}^{2}}+{{b}^{2}}+{{c}^{2}}\]
done
clear
C)
0
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 246) \[\left| \begin{matrix} 1/a & 1 & bc \\ 1/b & 1 & ca \\ 1/c & 1 & ab \\ \end{matrix} \right|\]is equal to
A)
1
done
clear
B)
0
done
clear
C)
\[(a+b+c)\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 247) If\[{{A}^{3}}=I\]for any matrix A, then\[{{A}^{-1}}\]is equal to
A)
\[{{A}^{2}}\]
done
clear
B)
A
done
clear
C)
\[{{A}^{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 248) For any two square matrices A and\[B,{{(AB)}^{-1}}\]is equal to
A)
\[{{A}^{-1}}{{B}^{-1}}\]
done
clear
B)
\[{{B}^{-1}}{{A}^{-1}}\]
done
clear
C)
\[{{(BA)}^{-1}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 249) For any matrix A, A (adj A) is equal to
A)
\[A\]
done
clear
B)
\[|A|{{I}_{n}}\]
done
clear
C)
\[|A|\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 250) If\[a,b,c\]are in GP, then\[{{\log }_{a}}x,{{\log }_{b}}x\]and\[{{\log }_{c}}x\]are in
A)
AP
done
clear
B)
GP
done
clear
C)
HP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 251) If\[a,b,c,d,e\]are in AP, then the value of\[a-4b+6c-4d+e\]is equal to
A)
1
done
clear
B)
2
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 252) If first term of a GP is double the sum of all consecutive terms starting from second term, then the common ratio is
A)
\[\frac{2}{5}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[\frac{1}{4}\]
done
clear
View Answer play_arrow
question_answer 253) Sum of the series\[{{11}^{3}}+{{12}^{3}}+...\text{ +}{{20}^{3}}\]is
A)
odd and divisible by 5
done
clear
B)
divisible by 10
done
clear
C)
odd and not divisible by 5
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 254) \[\left| \begin{matrix} 1 & 1 & 1 \\ 1 & \omega & {{\omega }^{2}} \\ 1 & {{\omega }^{2}} & \omega \\ \end{matrix} \right|\]is equal to
A)
0
done
clear
B)
\[3\sqrt{3}i\]
done
clear
C)
\[-3\sqrt{3}i\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 255) If\[{{a}^{2}}+{{b}^{2}}+{{c}^{2}}=1,\]then range of\[ab+bc+ca\]is
A)
\[\left[ -\frac{1}{2},\infty \right)\]
done
clear
B)
\[[0,\infty )\]
done
clear
C)
\[\left[ -\frac{1}{2},1 \right]\]
done
clear
D)
\[[1,\infty )\]
done
clear
View Answer play_arrow
question_answer 256) Range of the function\[\frac{x+2}{|x+2|}\]is
A)
\[\{-1,0,1\}\]
done
clear
B)
\[\{-1,1\}\]
done
clear
C)
\[[-1,1]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 257) Range of\[\frac{x}{3}\]is
A)
\[[-1,1]\]
done
clear
B)
\[\left[ -\frac{1}{3},\frac{1}{3} \right]\]
done
clear
C)
[0,2]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 258) Domain of the function\[{{\sin }^{-1}}\left( {{\log }_{2}}\frac{x}{2} \right)\]is
A)
\[\left[ \frac{1}{2},\frac{1}{4} \right]\]
done
clear
B)
\[[-1,3]\]
done
clear
C)
\[[1,4]\]
done
clear
D)
\[[0,1]\]
done
clear
View Answer play_arrow
question_answer 259) \[^{47}{{C}_{4}}+\sum\limits_{i=1}^{5}{^{52-i}{{C}_{3}}}\]is equal to
A)
\[^{50}{{C}_{4}}\]
done
clear
B)
\[^{50}{{C}_{4}}\]
done
clear
C)
\[^{10}{{C}_{5}}\]
done
clear
D)
\[^{52}{{C}_{6}}\]
done
clear
View Answer play_arrow
question_answer 260) Coefficient of the middle term in the expansion
A)
\[^{10}{{C}_{4}}\]
done
clear
B)
\[{{-}^{10}}{{C}_{4}}\]
done
clear
C)
\[^{10}{{C}_{5}}\]
done
clear
D)
\[^{10}{{C}_{6}}\]
done
clear
View Answer play_arrow
question_answer 261) If the coefficient of second, third and fourth terms in the expansion of\[{{(1+x)}^{n}}\]are in AP, then n is equal to
A)
7, 5
done
clear
B)
5, 4
done
clear
C)
4, 2
done
clear
D)
3, 6
done
clear
View Answer play_arrow
question_answer 262) If\[y=\sqrt{x+\sqrt{x+\sqrt{x+......\infty }}},\]then-y is equal to
A)
\[\frac{x}{2y-1}\]
done
clear
B)
\[\frac{1}{2y-1}\]
done
clear
C)
\[\frac{2}{y-1}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 263) Maximum value of the function\[f(x)=\frac{\log x}{x}\]in the interval\[0<x<\infty \]is
A)
e
done
clear
B)
\[\sqrt{e}\]
done
clear
C)
\[\frac{1}{e}\]
done
clear
D)
\[\frac{2}{e}\]
done
clear
View Answer play_arrow
question_answer 264) \[\int{\frac{dx}{\sqrt{x}(1+x)}}\]is equal to
A)
\[{{\tan }^{-1}}\sqrt{x}+c\]
done
clear
B)
\[2{{\tan }^{-1}}\sqrt{x}+c\]
done
clear
C)
\[2{{\tan }^{-1}}x+c\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 265) \[\int_{0}^{\pi /2}{\log \tan xdx}\]is equal to
A)
\[0\]
done
clear
B)
\[\pi \]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
question_answer 266) If\[\overrightarrow{a},\overrightarrow{b},\overrightarrow{c}\]are the position vectors of a\[\Delta ABC,\] then\[\overrightarrow{AB}+\overrightarrow{BC}+\overrightarrow{CA}\]is equal to
A)
\[\overrightarrow{0}\]
done
clear
B)
\[2\text{ }\overrightarrow{AB}\]
done
clear
C)
\[2\text{ }\overrightarrow{BA}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 267) The point (in first quadrant) on which the slope of the normal to the curve\[{{x}^{3}}=8{{a}^{2}}y\]is, \[-2/3,\] is
A)
(2a, a)
done
clear
B)
\[(-2a,a)\]
done
clear
C)
(a, 2a)
done
clear
D)
\[(a,-2a)\]
done
clear
View Answer play_arrow
question_answer 268) \[\int_{a}^{2a}{f(x)}\,dx\] is equal to
A)
\[2\int_{a}^{2a}{f(x)}\,dx\]
done
clear
B)
0
done
clear
C)
\[\int_{a}^{a}{f(x)}\,dx+\int_{0}^{2a}{f(2a-x)dx}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 269) If\[2\hat{i}-\hat{j}+\hat{k},\hat{i}+2\hat{j}-3\hat{k}\]and\[3\hat{i}+p\hat{j}+5\hat{k}\]are coplanar, then p is equal to
A)
1
done
clear
B)
-2
done
clear
C)
4
done
clear
D)
-4
done
clear
View Answer play_arrow
question_answer 270) Domain of the function\[\frac{1}{\sqrt{(x+2)(x-1)}}\]is
A)
\[(-\infty ,-2)\cup (1,\infty )\]
done
clear
B)
\[R-[-1,1]\]
done
clear
C)
\[(-\infty ,-2)\cup (2,\infty )\]
done
clear
D)
\[(-\infty ,1)\cup (2,\infty )\]
done
clear
View Answer play_arrow
question_answer 271) If three dice are thrown together, then the probability of getting same digit on all the dice
A)
\[\frac{1}{6}\]
done
clear
B)
\[\frac{3}{36}\]
done
clear
C)
\[\frac{1}{36}\]
done
clear
D)
\[\frac{2}{3}\]
done
clear
View Answer play_arrow
question_answer 272) If a dice is thrown twice, then the probability of getting 5 in first attempt only
A)
\[\frac{1}{36}\]
done
clear
B)
\[\frac{2}{36}\]
done
clear
C)
\[\frac{5}{36}\]
done
clear
D)
\[\frac{31}{36}\]
done
clear
View Answer play_arrow
question_answer 273) If two dice are thrown together, then probability of getting 7 as the sum, is
A)
\[\frac{1}{36}\]
done
clear
B)
\[\frac{11}{36}\]
done
clear
C)
\[\frac{2}{36}\]
done
clear
D)
\[\frac{1}{36}\]
done
clear
View Answer play_arrow
question_answer 274) A coin is tossed n times, then the probability of getting head odd number of times is
A)
\[\frac{1}{4}\]
done
clear
B)
\[{{\left( \frac{1}{2} \right)}^{n}}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[\frac{3}{8}\]
done
clear
View Answer play_arrow
question_answer 275) From the positive integers, four integers are selected randomly and then multiplied, then the probability of getting the digit 1, 3, 5 or 7 at the first place is
A)
\[\frac{16}{625}\]
done
clear
B)
\[\frac{8}{625}\]
done
clear
C)
\[\frac{10}{17}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 276) 15 persons shaked their hands with one another in a room. Total number of shake hand is
A)
90
done
clear
B)
105
done
clear
C)
110
done
clear
D)
115
done
clear
View Answer play_arrow
question_answer 277) The value of x for which \[|x-1|+|x-2|+|x-3|\ge 6,\]is
A)
\[0\le x\le 4\]
done
clear
B)
\[x\le -2\]and \[x\le 4\]
done
clear
C)
\[x\le 0\]and\[x\ge 4\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 278) \[a+ib<c+id\]is true only when
A)
\[a=0,c=0\]
done
clear
B)
\[a=0,d=0\]
done
clear
C)
\[b=0,c=0\]
done
clear
D)
\[b=0,d=0\]
done
clear
View Answer play_arrow
question_answer 279) If\[{{z}_{1}},{{z}_{2}}\]are any two complex numbers, then
A)
\[|{{z}_{1}}+{{z}_{2}}|\ge |{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
B)
\[|{{z}_{1}}+{{z}_{2}}|>|{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
C)
\[|{{z}_{1}}+{{z}_{2}}|\le |{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
D)
\[|{{z}_{1}}+{{z}_{2}}|=|{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
View Answer play_arrow
question_answer 280) \[\int{\sqrt{\frac{1+x}{1-x}}}dx\]is equal to
A)
\[-{{\sin }^{-1}}x\sqrt{1-{{x}^{2}}}+c\]
done
clear
B)
\[{{\sin }^{-1}}x-\sqrt{{{x}^{2}}-1}+c\]
done
clear
C)
\[{{\sin }^{-1}}x-\sqrt{1-{{x}^{2}}}+c\]
done
clear
D)
\[-{{\sin }^{-1}}x-\sqrt{{{x}^{2}}-1}+c\]
done
clear
View Answer play_arrow
question_answer 281) Equation of tangent to the curve\[{{x}^{2/3}}+{{y}^{2/3}}={{a}^{2/3}}\]at the point\[({{x}_{1}},{{y}_{1}})\]is
A)
\[xx_{1}^{1/3}+yy_{1}^{1/3}={{a}^{2/3}}\]
done
clear
B)
\[\frac{x}{{{x}^{1/3}}}+\frac{y}{{{y}^{1/3}}}={{a}^{2/3}}\]
done
clear
C)
\[x_{1}^{2/3}+yy_{1}^{2/3}={{a}^{1/3}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 282) If\[y=|x-1|+|x-3|,\]then the value of\[\frac{dy}{dx}\]at \[x=2\]is
A)
1
done
clear
B)
0
done
clear
C)
\[-1\]
done
clear
D)
does not exist
done
clear
View Answer play_arrow
question_answer 283) \[\frac{{{d}^{n}}}{d{{x}^{n}}}(\log x)\]is equal to
A)
\[\frac{{{(-1)}^{n-1}}(n-1)!}{{{x}^{n}}}\]
done
clear
B)
\[\frac{{{(-1)}^{n}}(n-1)!}{{{x}^{n-1}}}\]
done
clear
C)
\[\frac{{{(-1)}^{n}}n!}{{{x}^{n-1}}}\]
done
clear
D)
\[\frac{{{(-1)}^{n}}(n-1)!}{{{x}^{n-1}}}\]
done
clear
View Answer play_arrow
question_answer 284) If the vectors\[\overrightarrow{AB}=3\hat{i}+5\hat{j}+4\hat{k}\]and\[\overrightarrow{Ac}=5\hat{i}-5\hat{j}+2\hat{k}\]are the sides of the\[\Delta ABC,\]then length of median passing from A is
A)
\[\sqrt{3}\]units
done
clear
B)
\[2\sqrt{5}\]units
done
clear
C)
5 units
done
clear
D)
10 units
done
clear
View Answer play_arrow
question_answer 285) If the lines\[3x-4y+4=0\]and\[6x-8y-7=0\]are the tangents of a circle, then radius of the circle is
A)
\[\frac{3}{2}\]
done
clear
B)
\[\frac{3}{4}\]
done
clear
C)
\[\frac{1}{10}\]
done
clear
D)
\[\frac{1}{20}\]
done
clear
View Answer play_arrow
question_answer 286) Parametric coordinates of\[{{x}^{2}}=4ay\]are
A)
\[(a{{t}^{2}},2at)\]
done
clear
B)
\[(a{{t}^{2}},-2at)\]
done
clear
C)
\[(-2at,a{{t}^{2}})\]
done
clear
D)
\[(2at,a{{t}^{2}})\]
done
clear
View Answer play_arrow
question_answer 287) Cofactors of the elements of second row of the determinant \[\left| \begin{matrix} 2 & -1 & 4 \\ 4 & 2 & -3 \\ 1 & 1 & 2 \\ \end{matrix} \right|\]are
A)
5, 1, 8
done
clear
B)
6, 0, -3
done
clear
C)
5, 6, 4
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 288)
If \[A=\left[ _{-1}^{4}\,\,_{1}^{2} \right]\,\,,\] then \[(A-2I)(A-3I)\] is equal to
A)
\[{{A}^{2}}+6I\]
done
clear
B)
\[I\]
done
clear
C)
null matrix
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 289) If\[A=\left[ \begin{matrix} 4 & 1 \\ 3 & 2 \\ \end{matrix} \right],\]then\[{{A}^{2}}-6A\]is equal to
A)
\[3I\]
done
clear
B)
\[5I\]
done
clear
C)
\[-5I\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 290) If the points\[(x+1,2),(1,x+2)\]and \[\left( \frac{1}{x+1},\frac{2}{x+1} \right)\]are collinear, then\[x\]is equal to
A)
2
done
clear
B)
4
done
clear
C)
\[-4\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 291) If\[{{x}_{n}}=\cos \left( \frac{\pi }{{{2}^{n}}}+i\sin \frac{\pi }{{{2}^{n}}} \right),n=1,2,3.....\]then the value\[{{x}_{1}}.{{x}_{2}}.{{x}_{3}}.....\infty \]is
A)
1
done
clear
B)
\[-1\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 292) The number of ways to make a team of 10 players out of 22 players when 4 players are always in the team and 5 players are never in the team
A)
\[^{14}{{C}_{4}}\]
done
clear
B)
\[^{14}{{C}_{6}}\]
done
clear
C)
\[^{19}{{C}_{6}}\]
done
clear
D)
\[^{13}{{C}_{6}}\]
done
clear
View Answer play_arrow
question_answer 293) Equation\[\frac{{{x}^{2}}}{2-r}+\frac{{{y}^{2}}}{r-5}+1=0\]represents an ellipse, if
A)
\[r>5\]
done
clear
B)
\[r>2\]
done
clear
C)
\[2<r<5\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 294) If equation of latusrectum of a parabola is \[x+y=8\]and equation of tangent at its vertex is\[x+y=12,\]then length of its latusrectum is
A)
\[4\sqrt{2}\]
done
clear
B)
\[2\sqrt{2}\]
done
clear
C)
\[8\]
done
clear
D)
\[8\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 295) If area of the circle\[4{{x}^{2}}+4{{y}^{2}}-8x+16y+k=0\]is\[9\pi \]sq units, then the value of k is
A)
4
done
clear
B)
\[10\]
done
clear
C)
\[-16\]
done
clear
D)
±16
done
clear
View Answer play_arrow
question_answer 296) A rod of length 7 is kept with the support of a wall and floor of a room. If rod starts slipping, then the locus of its midpoint is
A)
a straight line
done
clear
B)
a circle
done
clear
C)
a parabola
done
clear
D)
an ellipse
done
clear
View Answer play_arrow
question_answer 297) Two points on the curve\[y={{x}^{2}}+2x\]with abscissae 1 and 3, slope of the line joining these two points
A)
3
done
clear
B)
5
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 298) \[si{{n}^{6}}A+co{{s}^{6}}A+3si{{n}^{2}}Aco{{s}^{2}}A\]is equal to
A)
3
done
clear
B)
2
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 299) The equation which has the roots\[\frac{1}{3+\sqrt{2}}\]and\[\frac{1}{3-\sqrt{2}}\]is
A)
\[7{{x}^{2}}-6x+1=0\]
done
clear
B)
\[6{{x}^{2}}-7x+1=0\]
done
clear
C)
\[{{x}^{2}}-6x+7=0\]
done
clear
D)
\[{{x}^{2}}-7x+6=0\]
done
clear
View Answer play_arrow
question_answer 300) If\[f(x)=\frac{2\sqrt{x+4}}{\sin 2x},(x\ne 0)\]is continuous at \[x=0,\]then\[f(0)\]is equal to
A)
1/4
done
clear
B)
\[-1/4\]
done
clear
C)
1/8
done
clear
D)
\[-1/8\]
done
clear
View Answer play_arrow
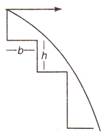
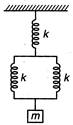
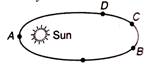
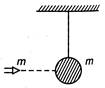
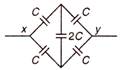
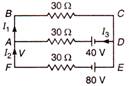
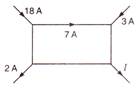
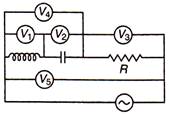
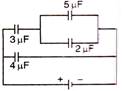
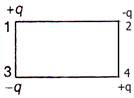
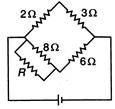
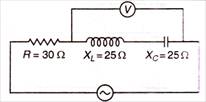
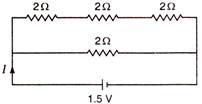
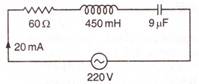
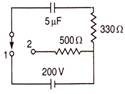
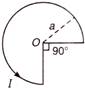
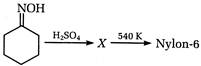 X, will be
X, will be
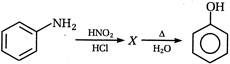 Here X, will be
Here X, will be
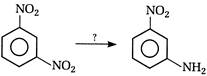 Above conversion is done by
Above conversion is done by
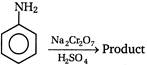
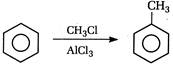
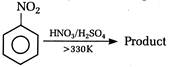
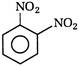
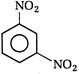
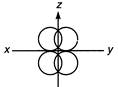
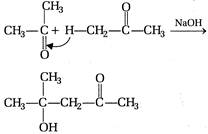 The above reaction is called
The above reaction is called