A) \[N{{i}^{2+}},T{{i}^{3+}}\]
B) \[S{{c}^{3+}},T{{i}^{3+}}\]
C) \[S{{c}^{3+}},C{{o}^{2+}}\]
D) \[N{{i}^{2+}},C{{u}^{+}}\]
Correct Answer: A
Solution :
\[_{28}Ni=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{8}},4{{s}^{2}}\]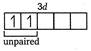 \[T{{i}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}}\] (1 unpaired electron) \[_{21}Sc=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}},4{{s}^{2}}\] \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\] (no unpaired electron) \[_{29}Cu=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] (no unpaired electron) Hence, in above ions,\[N{{i}^{2+}},\]and\[T{{I}^{3+}}\]ions are coloured ions in aqueous solution due to presence of unpaired electrons in d-subshell.
\[T{{i}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}}\] (1 unpaired electron) \[_{21}Sc=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}},4{{s}^{2}}\] \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\] (no unpaired electron) \[_{29}Cu=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] (no unpaired electron) Hence, in above ions,\[N{{i}^{2+}},\]and\[T{{I}^{3+}}\]ions are coloured ions in aqueous solution due to presence of unpaired electrons in d-subshell.
You need to login to perform this action.
You will be redirected in
3 sec
