A) \[HCHO>C{{H}_{3}}CHO>C{{H}_{3}}COC{{H}_{3}}\]
B) \[C{{H}_{3}}CHO>HCHO>C{{H}_{3}}COC{{H}_{3}}\]
C) \[C{{H}_{3}}COC{{H}_{3}}>C{{H}_{3}}CHO>HCHO\]
D) \[C{{H}_{3}}CHO>C{{H}_{3}}COC{{H}_{3}}>HCHO\]
Correct Answer: A
Solution :
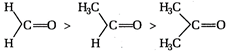 \[+I\]effect of\[C{{H}_{3}}\]group decreases reactivity of
\[+I\]effect of\[C{{H}_{3}}\]group decreases reactivity of You need to login to perform this action.
You will be redirected in
3 sec
