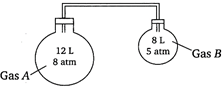
A) 8 and 5 atm
B) 9.6 and 4 atm
C) 4.8 and 2 atm
D) 6.4 and 4 atm
Correct Answer: C
Solution :
Moles of\[A,\,\,{{n}_{A}}=\frac{{{p}_{A}}{{V}_{A}}}{RT}=\frac{8\times 12}{RT}=\frac{96}{RT}\] Moles of\[B,\,\,{{n}_{B}}=\frac{{{p}_{B}}{{V}_{B}}}{RT}=\frac{8\times 5}{RT}=\frac{40}{RT}\] Total pressure\[\times \]total volume \[=({{n}_{A}}+{{n}_{B}})\times RT\] \[p\times (12+8)=\frac{1}{RT}(96+40)RT\] \[p=6.8\] \[\therefore \]Partial pressure of\[A=p\times \]mole fraction of\[A\] \[=6.8\times \left( \frac{96}{RT}/\frac{96+40}{RT} \right)\] \[=4.8\,\,\,atm\] \[\therefore \]Partial pressure of\[B=p\times \]mole fraction of\[B\] \[=6.8\times \left( \frac{40}{RT}/\frac{96+40}{RT} \right)\] \[=2\,\,\,atm\]You need to login to perform this action.
You will be redirected in
3 sec
