A) It is paramagnetic due to the presence of 4 unpaired electrons
B) It has coordination number of 6
C) It is outer orbital complex
D) It involves\[{{d}^{2}}s{{p}^{3}}\]hybridisation
Correct Answer: D
Solution :
In\[{{[Co{{(F)}_{6}}]}^{3-}}\], \[Co\]is in\[+3\]oxidation state.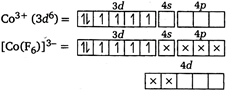 \[{{[Co{{(F)}_{6}}]}^{3-}}\]is an outer orbital complex showing\[s{{p}^{3}}{{d}^{2}}\]hybridisation due to the presence of weak field ligand. It is paramagnetic due to the presence of four unpaired electrons.
\[{{[Co{{(F)}_{6}}]}^{3-}}\]is an outer orbital complex showing\[s{{p}^{3}}{{d}^{2}}\]hybridisation due to the presence of weak field ligand. It is paramagnetic due to the presence of four unpaired electrons.
You need to login to perform this action.
You will be redirected in
3 sec
