A) \[\frac{9}{4}\]
B) \[\frac{4}{9}\]
C) \[\frac{3}{2}\]
D) \[\frac{7}{3}\]
Correct Answer: B
Solution :
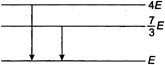
Transition from\[4E\]to\[E\] \[(4E-E)=\frac{hc}{{{\lambda }_{1}}}\Rightarrow {{\lambda }_{1}}=\frac{hc}{3E}\] ... (i) Transition from\[\frac{7}{3}E\]to\[E\] \[\left( \frac{7}{3}E-E \right)=\frac{hc}{{{\lambda }_{2}}}\Rightarrow {{\lambda }_{2}}=\frac{3hc}{4E}\] ? (ii) From Eq. (i) and (ii) \[\frac{{{\lambda }_{1}}}{{{\lambda }_{2}}}=\frac{4}{9}\]You need to login to perform this action.
You will be redirected in
3 sec
