A) \[N{{H}_{3}}<P{{H}_{3}}<As{{H}_{3}}-acidic\]
B) \[Li<Be<B<C-1st\,\,IP\]
C) \[A{{l}_{2}}{{O}_{3}}<MgO<N{{a}_{2}}O<{{K}_{2}}O-basic\]
D) \[L{{i}^{+}}<N{{a}^{+}}<{{K}^{+}}<C{{s}^{+}}-ionic\,\,radius\]
Correct Answer: B
Solution :
\[Li,\,\,Be,\,\,B\]and \[C\] are present in Und period. In a period from left to right ionisation potential increases. \[\xrightarrow[Li\,\,Be\,\,B\,\,C]{IP\,\,increases}\] * But in case of\[Be\] and\[B\], Be has higher \[IP\] than \[B\] due to stable configuration of\[Be\] \[_{4}Be=1{{s}^{2}},\,\,2{{s}^{2}}\]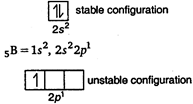 So, the correct order of IP of given elements is- \[Li<B<Be<C\]
So, the correct order of IP of given elements is- \[Li<B<Be<C\]
You need to login to perform this action.
You will be redirected in
3 sec
