A) \[ds{{p}^{2}}\]
B) \[s{{p}^{3}}d\]
C) \[s{{p}^{3}}{{d}^{2}}\]
D) \[s{{p}^{3}}{{d}^{3}}\]
Correct Answer: C
Solution :
Electronic configuration of\[Xe\]in ground state: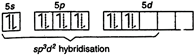 Note: The expected geometry of \[Xe{{F}_{4}}\] is octahedral. On account of the fact that \[lp-lp\] repulsion \[>lp-bp\] repulsion, there is some distortion in the shape of the molecule. Thus \[Xe{{F}_{4}}\] has distorted octahedral geometry with two lone pair of electrons. In other words, it has a square planar geometry.
Note: The expected geometry of \[Xe{{F}_{4}}\] is octahedral. On account of the fact that \[lp-lp\] repulsion \[>lp-bp\] repulsion, there is some distortion in the shape of the molecule. Thus \[Xe{{F}_{4}}\] has distorted octahedral geometry with two lone pair of electrons. In other words, it has a square planar geometry.
You need to login to perform this action.
You will be redirected in
3 sec
