question_answer 1) In the Davisson and Germer experiment, the velocity of electrons emitted from the electron gun can be increased by
A)
increasing the filament current
done
clear
B)
decreasing the filament current
done
clear
C)
decreasing the potential difference between the anode and filament
done
clear
D)
increasing the potential difference between the anode and filament
done
clear
View Answer play_arrow
A)
(iii), (ii) and (i)
done
clear
B)
(iii), (ii) and (iv)
done
clear
C)
(ii), (iv) and (iii)
done
clear
D)
(ii), (iii) and (iv)
done
clear
View Answer play_arrow
question_answer 3) A radioactive nucleus of mass M emits a photon of frequency v and the nucleus recoils. The recoil energy will be
A)
\[{{h}^{2}}{{v}^{2}}/2M{{c}^{2}}\]
done
clear
B)
zero
done
clear
C)
\[hv\]
done
clear
D)
\[M{{c}^{2}}-hv\]
done
clear
View Answer play_arrow
question_answer 4) A particle moves in a circle of radius 5 cm with constant speed and time period 0.2 us. The acceleration of the particle is
A)
\[25\,m/{{s}^{2}}\]
done
clear
B)
\[36\,m/{{s}^{2}}\]
done
clear
C)
\[5\,m/{{s}^{2}}\]
done
clear
D)
\[15\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 5) The power obtained in a reactor using \[{{U}^{235}}\] disintegration is 1000 kW. The mass decay of \[{{U}^{235}}\] per hour is
A)
\[20\mu g\]
done
clear
B)
\[40\mu g\]
done
clear
C)
\[1\mu g\]
done
clear
D)
\[10\mu g\]
done
clear
View Answer play_arrow
question_answer 6) The half-life of a radioactive isotope X is 50 yr. It decays to another element Y which is stable. The two elements X and Y were found to be in the ratio of 1 : 15 in a sample of a given rock. The age of the rock was estimated to be
A)
200 yr
done
clear
B)
250 yr
done
clear
C)
100 yr
done
clear
D)
150 yr
done
clear
View Answer play_arrow
question_answer 7) A parallel plate condenser has a uniform electric field E (V/m) in the space between the plates. If the distance between the plates is d(m) and area of each plate is \[A({{m}^{2}})\] the energy (joule) stored in the condenser is
A)
\[\frac{1}{2}{{\varepsilon }_{0}}{{E}^{2}}\]
done
clear
B)
\[{{\varepsilon }_{0}}EAd\]
done
clear
C)
\[\frac{1}{2}{{\varepsilon }_{0}}{{E}^{2}}Ad\]
done
clear
D)
\[{{E}^{2}}Ad/{{\varepsilon }_{0}}\]
done
clear
View Answer play_arrow
question_answer 8) The instantaneous angular position of a point on a rotating wheel is given by the equation \[Q(\tau )=2{{\tau }^{3}}-6{{\tau }^{2}}\] The torque on the wheel becomes zero at
A)
\[\tau =0.5\,s\]
done
clear
B)
\[\tau =0.25\,s\]
done
clear
C)
\[\tau =2\,s\]
done
clear
D)
\[\tau =1\,s\]
done
clear
View Answer play_arrow
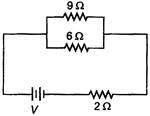
question_answer 9)
If power dissipated in the \[9\,\Omega \] resistor in the circuit shown is 36 W, the potential difference across the \[2\,\Omega \] resistor is
A)
8 V
done
clear
B)
10 V
done
clear
C)
2V
done
clear
D)
4 V
done
clear
View Answer play_arrow
question_answer 10) Two waves are represented by the equations \[{{y}_{1}}-a\sin (\omega t+kx+0.57)m\]and \[{{y}_{2}}-a\cos \,(\omega t+kx)m,\]in, where x is in metre and t in second. The phase difference between them is
A)
1.25 rad
done
clear
B)
1.57 rad
done
clear
C)
0.57 rad
done
clear
D)
1.0 rad
done
clear
View Answer play_arrow
question_answer 11) In photoelectric emission process from a metal of work function 1.8 eV, the kinetic energy of most energetic electrons is 0.5 eV. The corresponding stopping potential is
A)
1.3V
done
clear
B)
0.5V
done
clear
C)
2.3V
done
clear
D)
1.8V
done
clear
View Answer play_arrow
question_answer 12) A person of mass 60 kg is inside a lift of mass 940 kg and presses the button on control panel. The lift starts moving upwards with an acceleration \[1.0\,\,m/{{s}^{2}}\]. If \[g=10\,\,m/{{s}^{2}},\] the tension in the supporting cable is
A)
9680 N
done
clear
B)
11000 N
done
clear
C)
1200 N
done
clear
D)
8600 N
done
clear
View Answer play_arrow
question_answer 13) Out of the following functions representing motion of a particle which represents SHM I. \[y=\sin \omega t-\cos \omega t\] II. \[y={{\sin }^{3}}\omega t\] III. \[y=5\cos \left( \frac{3\pi }{4}-3\,\omega t \right)\] IV. \[y=1+\omega t+{{\omega }^{2}}{{t}^{2}}\]
A)
Only (IV) does not represent SHM
done
clear
B)
(I) and (III)
done
clear
C)
(I) and (II)
done
clear
D)
Only (I)
done
clear
View Answer play_arrow
question_answer 14) The moment of inertia of a thin uniform rod of mass M and length L about an axis passing through its mid-point and perpendicular to its length is \[{{I}_{0}}.\]Its moment of inertia about an axis passing through one of its ends and perpendicular to its length is
A)
\[{{I}_{0}}+M{{L}^{2}}/4\]
done
clear
B)
\[{{I}_{0}}+2M{{L}^{2}}\]
done
clear
C)
\[{{I}_{0}}+M{{L}^{2}}\]
done
clear
D)
\[{{I}_{0}}+M{{L}^{2}}/2\]
done
clear
View Answer play_arrow
question_answer 15) If a small amount of antimony is added to germanium crystal
A)
the antimony becomes an acceptor atom
done
clear
B)
there will be more free electrons than holes in the semiconductor
done
clear
C)
its resistance is increased
done
clear
D)
it becomes a p-type semiconductor
done
clear
View Answer play_arrow
question_answer 16) Sound waves travel at 350 m/s through a warn air and at 3500 m/s through brass. The wavelength of a 700 Hz acoustic wave as it enters brass from warm air
A)
increases by a factor 20
done
clear
B)
increases by a factor 10
done
clear
C)
decreases by a factor 20
done
clear
D)
decreases by a factor 10
done
clear
View Answer play_arrow
question_answer 17) Light of two different frequencies whose photons have energies 1 eV and 2.5 eV respectively illuminate a metallic surface whose work function is 0.5 eV successively. Ratio of maximum speeds of emitted electrons will be
A)
1 : 2
done
clear
B)
1 : 1
done
clear
C)
1 : 5
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 18) A current of 2 A flows through a \[2\,\Omega \] resistor when connected across a battery. The same battery supplies a current of 0.5 A when connected across a \[9\,\Omega \] resistor. The internal resistance of the battery is
A)
\[1/3\,\,\Omega \]
done
clear
B)
\[1/4\,\,\Omega \]
done
clear
C)
\[1\,\Omega \]
done
clear
D)
\[0.5\,\Omega \]
done
clear
View Answer play_arrow
question_answer 19) The electric and the magnetic field, associated with an electromagnetic wave, propagating along the +z-axis, can be represented by
A)
\[[\mathbf{E}={{E}_{0}}\mathbf{\hat{k}},\mathbf{B}={{B}_{0}}\mathbf{\hat{i}}]\]
done
clear
B)
\[[\mathbf{E}={{E}_{0}}\mathbf{\hat{j}}\,,\mathbf{B}={{B}_{0}}\mathbf{\hat{j}}\,]\]
done
clear
C)
\[[\mathbf{E}={{E}_{0}}\mathbf{\hat{j}}\,,\mathbf{B}={{B}_{0}}\mathbf{\hat{k}}\,]\]
done
clear
D)
\[[\mathbf{E}={{E}_{0}}\mathbf{\hat{i}}\,,\mathbf{B}={{B}_{0}}\mathbf{\hat{j}}\,]\]
done
clear
View Answer play_arrow
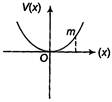
question_answer 20)
A particle of mass m is released from rest and follows a parabolic path as shown. Assuming that the displacement of the mass from the origin is small, which graph correctly depicts the position of the particle as a function of time?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 21) When 1kg of ice at \[{{0}^{\text{o}}}C\] melts to water at \[{{0}^{\text{o}}}C\], the resulting change in its entropy, taking latent heat of ice to be 80 cal/°C, is
A)
\[8\times {{10}^{4}}\,cal/K\]
done
clear
B)
\[80\,\,cal/K\]
done
clear
C)
\[293\,\,cal/K\]
done
clear
D)
\[273\,\,cal/K\]
done
clear
View Answer play_arrow
question_answer 22) A planet moving along an elliptical orbit is 28 closest to the sun at a distance \[{{r}_{1}}\] and farthest away at a distance of\[{{r}_{2}}.\] If\[{{v}_{1}}\] and \[{{r}_{2}}\] are the linear velocities at these points respectively, then the ratio\[\frac{{{v}_{1}}}{{{v}_{2}}}\] is
A)
\[{{r}_{2}}/{{r}_{1}}\]
done
clear
B)
\[{{({{r}_{2}}/{{r}_{1}})}^{2}}\]
done
clear
C)
\[{{r}_{1}}/{{r}_{2}}\]
done
clear
D)
\[{{({{r}_{1}}/{{r}_{2}})}^{2}}\]
done
clear
View Answer play_arrow
question_answer 23) A charge Q is enclosed by a Gaussian spherical surface of radius R. If the radius is doubled, then the outward electric flux will
A)
be reduced to half
done
clear
B)
remain the same
done
clear
C)
be doubled
done
clear
D)
increase four times
done
clear
View Answer play_arrow
question_answer 24) The rate of increase of thermo-emf with temperature at the neutral temperature of a thermocouple
A)
is zero
done
clear
B)
depends upon the choice of the two materials of the thermocouple
done
clear
C)
is negative
done
clear
D)
is positive
done
clear
View Answer play_arrow
question_answer 25) Fusion reaction takes place at high temperature because
A)
atoms get ionised at high temperature
done
clear
B)
kinetic energy is high enough to overcome the coulomb repulsion between nuclei
done
clear
C)
molecules break up at high temperature
done
clear
D)
nuclei break up at high temperature
done
clear
View Answer play_arrow
question_answer 26) A nucleus \[_{n}^{m}X\] emits one a-particle and two \[{{}^{\text{-}}}\] particles. The resulting nucleus is
A)
\[_{n}^{m-6}Z\]
done
clear
B)
\[_{n}^{m-4}X\]
done
clear
C)
\[_{n-2}^{m-4}Y\]
done
clear
D)
\[_{n-4}^{m-6}Z\]
done
clear
View Answer play_arrow
question_answer 27) There are four light-weight-rod samples A, B, C, D separately suspended by threads. A bar magnet is slowly brought near each sample and the following observations are noted (i) A is feebly repelled (ii) B is feebly attracted (iii) C is strongly attracted (iv) D remains unaffected Which one of the following is true?
A)
C is of a diamagnetic material
done
clear
B)
D is of a ferromagnetic material
done
clear
C)
A is of a non-magnetic material
done
clear
D)
B is of a paramagnetic material
done
clear
View Answer play_arrow
question_answer 28) During an isothermal expansion, a confined ideal gas does -150 J of work against its surroundings. This implies that
A)
300 J of heat has been added to the gas
done
clear
B)
no heat is transferred because the process is isothermal
done
clear
C)
150 J of heat has been added to the gas
done
clear
D)
150 J of heat has been removed from the gas
done
clear
View Answer play_arrow
question_answer 29) Photoelectric emission occurs only when the incident light has more than a certain minimum
A)
wavelength
done
clear
B)
intensity
done
clear
C)
frequency
done
clear
D)
power
done
clear
View Answer play_arrow
question_answer 30) Electrons used in an electron microscope are accelerated by a voltage of 25 kV. If the voltage is increased to 100 kV then the de-Broglie wavelength associated with the electrons would
A)
decrease by 2 times
done
clear
B)
decrease by 4 times
done
clear
C)
increase by 4 times
done
clear
D)
increase by 2 times
done
clear
View Answer play_arrow
question_answer 31) In an AC circuit an alternating voltage \[e=200\sqrt{2}\sin \]100t volt is connected to a capacitor of capacity \[1\,\mu F\]. The \[rms\] value of the current in the circuit is
A)
100 mA
done
clear
B)
200 mA
done
clear
C)
20 mA
done
clear
D)
10 mA
done
clear
View Answer play_arrow
question_answer 32) A boy standing at the top of a tower of 20 m height drops a stone. Assuming \[g=10\,m{{s}^{-2}}\], the velocity with which it hits the ground is
A)
20 m/s
done
clear
B)
40 m/s
done
clear
C)
5 m/s
done
clear
D)
10 m/s
done
clear
View Answer play_arrow
question_answer 33) A body projected vertically from the earth reaches a height equal to earth's radius before returning to the earth. The power exerted by the gravitational force is greatest
A)
at the instant just before the body hits the earth
done
clear
B)
it remains constant all through
done
clear
C)
at the instant just after the body is projected
done
clear
D)
at the highest position of the body
done
clear
View Answer play_arrow
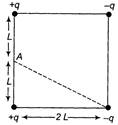
question_answer 34)
Pour electric charges +q, + q, - q and - q are placed at the comers of a square of side 2L (see figure). The electric potential at point A, mid-way between the two charges + q and + q, is
A)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{2a}{L}\left( 1+\frac{1}{\sqrt{5}} \right)\]
done
clear
B)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{2a}{L}\left( 1-\frac{1}{\sqrt{5}} \right)\]
done
clear
C)
zero
done
clear
D)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{2q}{L}\left( 1+\sqrt{5} \right)\]
done
clear
View Answer play_arrow
question_answer 35) The dimensions of \[{{({{\mu }_{0}}{{\varepsilon }_{0}})}^{-1/2}}\]
A)
\[[{{L}^{-1}}T]\]
done
clear
B)
\[[L{{T}^{-1}}]\]
done
clear
C)
\[[{{L}^{-1/2}}\,{{T}^{1/2}}]\]
done
clear
D)
\[[{{L}^{1/2}}\,{{T}^{-1/2}}]\]
done
clear
View Answer play_arrow
question_answer 36) A body is moving with velocity 30 m/s towards east. After 10 s its velocity becomes 40 m/s towards north. The average acceleration of the body is
A)
\[7\,m/{{s}^{2}}\]
done
clear
B)
\[\sqrt{7}\,m/{{s}^{2}}\]
done
clear
C)
\[5\,m/{{s}^{2}}\]
done
clear
D)
\[1\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 37) A biconvex lens has a radius of curvature of magnitude 20 cm. Which one of the following options describe best the image formed of an object of height 2 cm placed 30 cm from the lens?
A)
Virtual, upright, height = 0.5 cm
done
clear
B)
Real, inverted, height = 4 cm
done
clear
C)
Real, inverted, height = 1 cm
done
clear
D)
Virtual, upright, height = 1 cm
done
clear
View Answer play_arrow
question_answer 38) The wavelength of the first line of Lyman series for hydrogen atom is equal to that of the second line of Balmer series for a hydrogen like ion. The atomic number Z of hydrogen like ion is
A)
4
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 39) Which of the following is not due to total internal reflection?
A)
Difference between apparent and real depth of a pond
done
clear
B)
Mirage on hot summer days
done
clear
C)
Brilliance of diamond
done
clear
D)
Working of optical fibre
done
clear
View Answer play_arrow
question_answer 40) The potential energy of a system increase if work is done
A)
by the system against a conservative force
done
clear
B)
by the system against a neoconservative force
done
clear
C)
upon the system by a conservative force
done
clear
D)
upon the system by a neoconservative force
done
clear
View Answer play_arrow
question_answer 41) In forward biasing of the p-n junction
A)
the positive terminal of the battery is connected to n-side and the depletion region becomes thin
done
clear
B)
the positive terminal of the battery is connected to n-side and the depletion region becomes thick
done
clear
C)
the positive terminal of the battery is connected to p-side and the depletion region become thin
done
clear
D)
the positive terminal of the battery is connected to p-side and the depletion region becomes thick
done
clear
View Answer play_arrow
question_answer 42) A missile is fired for maximum range with a initial velocity of 20 m/s. If \[g=10\,\,m/{{s}^{2}},\] the range of the missile is
A)
50 m
done
clear
B)
60 m
done
clear
C)
20 m
done
clear
D)
40 m
done
clear
View Answer play_arrow
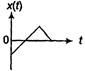
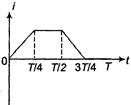
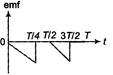
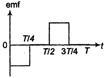
question_answer 43)
The current; in a coil varies with time as shown in the figure. The variation of induced emf with time would be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 44) An AC voltage is applied to a resistance R and an inductor L in series. If R and the inductive reactance are both equal to \[3\,\Omega ,\]the phase difference between the applied voltage and the current in the circuit is
A)
\[\pi /4\]
done
clear
B)
\[\pi /2\]
done
clear
C)
zero
done
clear
D)
\[\pi /6\]
done
clear
View Answer play_arrow
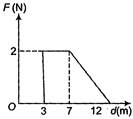
question_answer 45)
Force F on a particle moving in a straight line varies with distance d as shown in the figure. The work done on the particle during its displacement of 12 m is
A)
21 J
done
clear
B)
26 J
done
clear
C)
13 J
done
clear
D)
18 J
done
clear
View Answer play_arrow
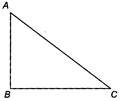
question_answer 46)
A current carrying closed loop in the form of a right angle isosceles triangle ABC is placed in a uniform magnetic field acting along AB. If the magnetic force on the arm BC is F, the force on the arm AC is
A)
\[-\mathbf{F}\]
done
clear
B)
\[\mathbf{F}\]
done
clear
C)
\[\sqrt{2}\mathbf{F}\]
done
clear
D)
\[-\sqrt{2}\mathbf{F}\]
done
clear
View Answer play_arrow
question_answer 47) The decreasing order of wavelength of infrared, microwave, ultraviolet and gamma rays is
A)
gamma rays, ultraviolet, infrared, microwaves
done
clear
B)
(b microwaves, gamma rays, infrared, ultraviolet
done
clear
C)
infrared, microwave, ultraviolet, gamma rays
done
clear
D)
microwave infrared, ultraviolet, gamma rays
done
clear
View Answer play_arrow
question_answer 48) A transistor is operated in common emitter configuration at \[{{V}_{C}}=2\,V\] such that a change in the base current from 100 \[\text{A}\] to 300 \[\text{A}\] produces a change in the collector current from 10 mA to 20 mA. The current gain is
A)
75
done
clear
B)
100
done
clear
C)
25
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 49) A body of mass M hits normally a rigid wall with velocity v and bounces back with the same velocity. The impulse experienced by the body is
A)
1.5 Mv
done
clear
B)
2 Mv
done
clear
C)
zero
done
clear
D)
Mv
done
clear
View Answer play_arrow
question_answer 50) A uniform electric field and a uniform magnetic field are acting along the same direction in a certain region. If an electron is projected in the region such that its velocity is pointed along the direction of fields, then the electron
A)
speed will decrease
done
clear
B)
speed will increase
done
clear
C)
will turn towards left of direction of motion
done
clear
D)
will turn towards right of direction a motion
done
clear
View Answer play_arrow
question_answer 51) Standard electrode potential of three metals X, Y and Z are -1.2 V, + 0.5 V and -3.0 V respectively. The reducing power of these metal will be
A)
Y > X > Z
done
clear
B)
Z > X > Y
done
clear
C)
X > Y > Z
done
clear
D)
Y > Z > X
done
clear
View Answer play_arrow
question_answer 52) Considering the state of hybridisation of carbon atoms, find out the molecule among the following which is linear.
A)
\[C{{H}_{3}}-C\equiv C-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{2}}=CH-C{{H}_{2}}-C\equiv CH\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 53) Clemmensen reduction of a ketone is carried out in the presence of which of the following?
A)
Zn-Hg with HCl
done
clear
B)
\[LiAl{{H}_{4}}\]
done
clear
C)
\[{{H}_{2}}\] and Pt as catalyst
done
clear
D)
Glycol with KOH
done
clear
View Answer play_arrow
question_answer 54) A gaseous mixture was prepared by taking equal moles of CO and \[{{N}_{2}}\]. If the total pressure of the mixture was found 1 atmosphere the partial pressure of the nitrogen \[({{N}_{2}})\] in the mixture is
A)
0.8 atm
done
clear
B)
0.9 atm
done
clear
C)
1 atm
done
clear
D)
0.5 atm
done
clear
View Answer play_arrow
question_answer 55) In the following reactions, (I) \[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{3}} & \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{CH}}}\,-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{OH} \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{C}{{\text{H}}_{\text{3}}}\xrightarrow[{}]{{{\text{H}}^{\text{+}}}\text{/Heat}}\] \[{{\left( \overset{\text{A}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)}^{\text{+}}}\left( \overset{\text{B}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)\] (ii)\[\text{A}\xrightarrow[\text{in}\,\text{absence}\,\text{of}\,\text{peroxide}]{\text{HBr,dark}}\] \[{{\left( \overset{\text{C}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)}^{\text{+}}}\left( \overset{\text{D}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)\] the major products (A) and (C) are respectively
A)
\[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{=}\,\text{CH}-\text{C}{{\text{H}}_{\text{3}}}\] and \[\text{C}{{\text{H}}_{\text{3}}}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{Br} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{CH}{{ }_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
B)
\[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{=}\,\text{CH}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,-\underset{\begin{smallmatrix} | \\ \text{Br} \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
C)
\[\text{C}{{\text{H}}_{2}}=\,\,\,\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,-\text{C}{{\text{H}}_{2}}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\text{C}{{\text{H}}_{\text{3}}}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{Br} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{2}}}\text{=}\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\underset{\begin{smallmatrix} | \\ \text{Br} \end{smallmatrix}}{\mathop{\text{C}{{\text{H}}_{\text{2}}}}}\,-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\,\text{CH}\,}}\,-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 56) If x is amount of adsorb ate and m is amount of adsorbent, which of the following relations is not related to adsorption process?
A)
\[\frac{x}{m}=f(T)\] at constant?
done
clear
B)
\[p=f(T)\] at constant \[\left( \frac{x}{m} \right)\]
done
clear
C)
\[\frac{x}{m}=p\times T\]
done
clear
D)
\[\frac{x}{m}=f\](p) at constant T
done
clear
View Answer play_arrow
question_answer 57) The freezing point depression constant for water is \[-{{1.86}^{o}}C\,{{m}^{-1}}.\]If 5.00 g \[N{{a}_{2}}S{{O}_{4}}\]is dissolved in \[45.0g\,{{H}_{2}}O,\] the freezing point is changed by -3.82°C. Calculate the van't Hoff factor for \[N{{a}_{2}}S{{O}_{4}}.\]
A)
2.63
done
clear
B)
3.11
done
clear
C)
0.381
done
clear
D)
2.05
done
clear
View Answer play_arrow
question_answer 58) Which of the two ions from the list given below, have the geometry that is explained by the same hybridisation of orbitals, \[NO_{2}^{-},NO_{3}^{-},NH_{2}^{-},NH_{4}^{+},SC{{N}^{-}}?\]
A)
\[NH_{4}^{-}\,and\,NO_{3}^{-}\]
done
clear
B)
\[SCN_{{}}^{-}\,and\,NH_{2}^{-}\]
done
clear
C)
\[NO_{2}^{-}\,and\,NH_{2}^{-}\]
done
clear
D)
\[NO_{2}^{-}\,and\,NH_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 59) The d-electron configurations of \[C{{r}^{2+}},M{{n}^{2+}},\] \[F{{e}^{2+}}\] and \[C{{o}^{2+}}\] are \[{{d}^{4}},{{d}^{5}},{{d}^{6}}\] and \[{{d}^{7}}\] respectively. Which one of the following will exhibit minimum paramagnetic behaviour? (At. no. Cr = 24, Mn = 25, Fe = 26, Co = 27)
A)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 60) For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order? (At. no. Cr = 24, Mn = 25, Fe = 26, Co = 27)
A)
Fe > Mn > Co > Cr
done
clear
B)
Co > Mn > Fe > Cr
done
clear
C)
Cr > Mn > Co > Fe
done
clear
D)
Mn > Fe > Cr > Co
done
clear
View Answer play_arrow
question_answer 61) The van't Hoff factor, i for a compound which undergoes dissociation in one solvent and association in other solvent is respectively.
A)
less than one and less than one
done
clear
B)
greater than one and less than one
done
clear
C)
greater than one and greater than one
done
clear
D)
less than one and greater than one
done
clear
View Answer play_arrow
question_answer 62) Which one of the following statements is not true regarding (+) lactose?
A)
(+) lactose is a \[\text{-}\]glycoside formed by the union of a molecule of D(+) glucose and a molecule of D(+) galactose
done
clear
B)
(+) lactose is a reducing sugar and does not exhibit mutarotation
done
clear
C)
(+) lactose,\[{{C}_{12}}{{H}_{22}}{{O}_{11}}\]contains 8-OH groups
done
clear
D)
On hydrolysis (+) lactose gives equal amount of D (+) glucose and D (+) galactose
done
clear
View Answer play_arrow
question_answer 63) If the \[{{\text{E}}^{\text{o}}}_{\text{cell}}\]for a given reaction has a negative value then which of the following gives the correct relationships for the values of \[{{\text{G}}^{\text{o}}}\] and \[{{\text{K}}_{\text{eq}}}\]?
A)
\[\Delta {{\text{G}}^{\text{o}}}\text{}\,\text{0;}\,{{\text{K}}_{\text{eq}}}>1\]
done
clear
B)
\[\Delta {{\text{G}}^{\text{o}}}\text{}\,\text{0;}\,{{\text{K}}_{\text{eq}}}<1\]
done
clear
C)
\[\Delta {{\text{G}}^{\text{o}}}>\,\text{0;}\,{{\text{K}}_{\text{eq}}}<1\]
done
clear
D)
\[\Delta {{\text{G}}^{\text{o}}}>\,\text{0;}\,{{\text{K}}_{\text{eq}}}>1\]
done
clear
View Answer play_arrow
question_answer 64) Which one of the following is employed as antihistamine?
A)
Diphenyl hydramine
done
clear
B)
Norethindrone
done
clear
C)
Omeprazole
done
clear
D)
Chloramphenicol
done
clear
View Answer play_arrow
question_answer 65) Which of the following elements is present as the impurity to the maximum extent in the pig iron?
A)
Carbon
done
clear
B)
Silicon
done
clear
C)
Phosphorus
done
clear
D)
Manganese
done
clear
View Answer play_arrow
question_answer 66) Of the following complex ions, which is diamagnetic in nature?
A)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
done
clear
B)
\[{{[CuC{{l}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Co{{F}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[NiC{{l}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 67) The electrode potentials for \[C{{u}^{2+}}(aq)+{{e}^{-}}\xrightarrow[{}]{{}}C{{u}^{+}}(aq)\]and \[C{{u}^{+}}(aq)+{{e}^{-}}\xrightarrow[{}]{{}}Cu(s)\] Are + 0.15 V and + 0.50 v respectively. The value of \[{{E}^{o}}_{C{{u}^{2+}}/Cu}\]will be
A)
0.325 V
done
clear
B)
0.650 V
done
clear
C)
0.150 V
done
clear
D)
0.500 V
done
clear
View Answer play_arrow
question_answer 68) If the enthalpy change for the transition of liquid water to steam is 30 kJ mol-1 at 27°C, the entropy change for the process would be
A)
\[\text{1}\text{.0J}\,\text{mo}{{\text{l}}^{\text{-1}}}\,{{\text{K}}^{\text{-1}}}\]
done
clear
B)
\[\text{0}\text{.1}\,\text{J}\,\text{mo}{{\text{l}}^{\text{-1}}}\,{{\text{K}}^{\text{-1}}}\]
done
clear
C)
\[100\,\text{J}\,\text{mo}{{\text{l}}^{\text{-1}}}\,{{\text{K}}^{\text{-1}}}\]
done
clear
D)
\[10\,\text{J}\,\text{mo}{{\text{l}}^{\text{-1}}}\,{{\text{K}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
question_answer 69) Which one of the following statements for the order of a reaction is incorrect?
A)
Order is not influenced by stoichiometric coefficient of the reactants
done
clear
B)
Order of reaction is sum of power to the concentration terms of reactants to express the rate of reaction
done
clear
C)
Order of reaction is always whole number
done
clear
D)
Order can be determined only experimentally
done
clear
View Answer play_arrow
question_answer 70) The correct order of increasing bond length of \[C-H,C-O,C-C\]and\[C=C\]is
A)
\[C-C<C=C<C-O<C-H\]
done
clear
B)
\[C-O<C-H<C-C<C=C\]
done
clear
C)
\[C-H<C-O<C-C<C=C\]
done
clear
D)
\[C-H<C=C<C-O<C-C\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following is least likely to behave as Lewis base?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[O{{H}^{-}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 72) Of the following which one is classified as polyester polymer?
A)
Bakelite
done
clear
B)
Melamine
done
clear
C)
Nylon-66
done
clear
D)
Terylene
done
clear
View Answer play_arrow
question_answer 73) A buffer solution is prepared in which the concentration of \[N{{H}_{3}}\] is 0.30 M and the concentration of \[\text{NH}_{\text{4}}^{\text{+}}\] is 0.20 M. If the equilibrium constant, \[{{K}_{b}}\] for \[N{{H}_{3}}\] equals \[1.8\times {{10}^{-5}},\] what is the pH of this solution? (log 2.7 =0.43)
A)
9.43
done
clear
B)
11.72
done
clear
C)
8.73
done
clear
D)
9.08
done
clear
View Answer play_arrow
question_answer 74) Standard electrode potential for \[\text{S}{{\text{n}}^{\text{4+}}}\text{/S}{{\text{n}}^{\text{2+}}}\] couple is +0.15 V and that for the \[C{{r}^{3+}}/Cr\] couple is -0.74. These two couples in their standard state are connected to make a cell. The cell potential will be
A)
+ 0.89 V
done
clear
B)
+ 0.18 V
done
clear
C)
+ 1.83 V
done
clear
D)
+ 1.199 V
done
clear
View Answer play_arrow
question_answer 75)
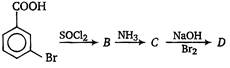
In a set of reactions, m-bromobenzoic acid gave a product D. Identify the product D.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 76) Name the type of the structure of silicate in which one oxygen atom of\[{{\text{ }\!\![\!\!\text{ Si}{{\text{O}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{4-}}}\]is shared?
A)
Sheet silicate
done
clear
B)
Pyrosilicate
done
clear
C)
Three dimensional silicate
done
clear
D)
Linear chain silicate
done
clear
View Answer play_arrow
question_answer 77)
A)
3-ethyl-4-ethenylheptane
done
clear
B)
3-ethyl-4-propylhex-5-ene
done
clear
C)
3-(1-ethyl propyl) hex-1-ene
done
clear
D)
4-ethyl-3-propylhex-1-ene
done
clear
View Answer play_arrow
question_answer 78)
What is the product obtained in the following reaction?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 79) Which of the following is correct option for free expansion of an ideal gas under adiabatic condition?
A)
\[q\ne 0,\Delta T=0,W=0\]
done
clear
B)
\[q=0,\Delta T=0,W=0\]
done
clear
C)
\[q=0,\Delta T<0,W\ne 0\]
done
clear
D)
\[q=0,\Delta T\ne 0,W=0\]
done
clear
View Answer play_arrow
question_answer 80) Enthalpy change for the reaction, \[4H(g)\xrightarrow[{}]{{}}2{{H}_{2}}(g)\]is ? 869.6 kJ The dissociation energy of H?H bond is
A)
\[-869.6\,kJ\]
done
clear
B)
\[+\,434.8\,kJ\]
done
clear
C)
\[+\,217.4\,kJ\]
done
clear
D)
\[-\,434.8\,kJ\]
done
clear
View Answer play_arrow
question_answer 81) Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively. The molecular mass of A is 49 u. Molecular mass of B will be
A)
12.25 u
done
clear
B)
6.50 u
done
clear
C)
25.00 u
done
clear
D)
50.00 u
done
clear
View Answer play_arrow
question_answer 82) The complexes \[[Co{{(N{{H}_{3}})}_{6}}][R{{(CN)}_{6}}]\] and \[[Cr{{(N{{H}_{3}})}_{6}}][Co{{(CN)}_{6}}]\] are the examples of which type of isomerism?
A)
Ionisation isomerism
done
clear
B)
Co-ordination isomerism
done
clear
C)
Geometrical isomerism
done
clear
D)
Linkage isomerism
done
clear
View Answer play_arrow
question_answer 83) Which of the following pairs of metals is purified by van Arkel method?
A)
Zr and Ti
done
clear
B)
Ag and Au
done
clear
C)
Ni and Fe
done
clear
D)
Ga and In
done
clear
View Answer play_arrow
question_answer 84) Which one is a nucleophilic substitution reaction among the following?
A)
\[\text{RCHO+R }\!\!'\!\!\text{ MaX}\xrightarrow[{}]{{}}\text{R}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{OH} \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{R}\]
done
clear
B)
\[\text{C}{{\text{H}}_{\text{3}}}-\text{C}{{\text{H}}_{\text{2}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{C}{{\text{H}}_{\text{2}}}\text{Br}\,\text{+}\,\text{N}{{\text{H}}_{\text{3}}}\xrightarrow[{}]{{}}\]\[\text{C}{{\text{H}}_{\text{3}}}-\text{C}{{\text{H}}_{\text{2}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{C}{{\text{H}}_{\text{2}}}\text{N}{{\text{H}}_{2}}\]
done
clear
C)
\[\text{C}{{\text{H}}_{\text{3}}}\text{CHO}\,\text{+}\,\text{HCN}\xrightarrow[{}]{{}}\text{C}{{\text{H}}_{\text{3}}}\text{CH(OH)CN}\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{3}}}-\text{CH}\,\text{=}\,\text{C}{{\text{H}}_{\text{2}}}+{{\text{H}}_{\text{2}}}\text{O}\xrightarrow[{}]{{{\text{H}}^{\text{+}}}}\]\[\text{C}{{\text{H}}_{\text{3}}}-\underset{\begin{smallmatrix} | \\ \text{OH} \end{smallmatrix}}{\mathop{\text{CH}\,}}\,-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 85) Which of the following compounds has the lowest melting point?
A)
\[CaB{{r}_{2}}\]
done
clear
B)
\[Ca{{I}_{2}}\]
done
clear
C)
\[Ca{{F}_{2}}\]
done
clear
D)
\[CaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 86) The total number of atomic orbitals in fourth energy level of an atom is
A)
16
done
clear
B)
32
done
clear
C)
4
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 87) Which one of the following is most reactive towards electrophonic reagent?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 88) By what factor does the average velocity of a gaseous molecule increase when the temperature (in Kelvin) is doubled?
A)
2.8
done
clear
B)
4.0
done
clear
C)
1.4
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 89) In Duma's method of estimation of nitrogen 0.35 g of an organic compound gave 55 mL of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be (Aqueous tension at 300 K = 15 mm)
A)
16.45
done
clear
B)
17.45
done
clear
C)
14.45
done
clear
D)
15.45
done
clear
View Answer play_arrow
question_answer 90) The complex,\[[Pt(Py)(N{{H}_{3}})BrCl]\]will have how many geometrical isomers?
A)
4
done
clear
B)
0
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 91) Mole fraction of the solute in a 1.00 molal aqueous solution is
A)
0.0177
done
clear
B)
0.0344
done
clear
C)
1.7700
done
clear
D)
0.1770
done
clear
View Answer play_arrow
question_answer 92) The value of \[\Delta H\]for the reaction \[{{X}_{2}}(g)+4{{Y}_{2}}(g)\rightleftharpoons 2X{{Y}_{4}}(g)\] is less than zero. Formation of\[X{{Y}_{4}}(g)\]will be favored at
A)
low pressure and low temperature
done
clear
B)
high temperature and low pressure
done
clear
C)
high pressure and low temperature
done
clear
D)
high temperature and high pressure
done
clear
View Answer play_arrow
question_answer 93) The Lassaigne's extract is boiled with cone\[\text{HN}{{\text{O}}_{\text{3}}}\]while testing for halogens. By doing so it
A)
helps in the precipitation of \[AgCl\]
done
clear
B)
increases the solubility product of \[AgCl\]
done
clear
C)
increases the concentration of \[NO_{3}^{-}\] ions
done
clear
D)
decomposes \[\text{N}{{\text{a}}_{\text{2}}}\text{S}\] and NaCN, if formed
done
clear
View Answer play_arrow
question_answer 94) For the reaction \[{{N}_{2}}(g)+{{O}_{2}}\]\[(g)2\]\[NO(g),\]the equilibrium constant is \[{{K}_{1}}\]. The equilibrium constant is \[{{K}_{2}}\] for the reaction \[2NO(g)+{{O}_{2}}(g)\]\[2N{{O}_{2}}(g).\]What is K for the reaction \[N{{O}_{2}}(g)\frac{1}{2}{{N}_{2}}(g)+{{O}_{2}}(g)\]?
A)
\[1/(4{{K}_{1}}{{K}_{2}})\]
done
clear
B)
\[{{[1/{{K}_{1}}{{K}_{2}}]}^{1/2}}\]
done
clear
C)
\[1/({{K}_{1}}{{K}_{2}})\]
done
clear
D)
\[1/(2{{K}_{1}}{{K}_{2}})\]
done
clear
View Answer play_arrow
question_answer 95) The energies E1 and E2 of two radiations are 25 eV and 50 eV respectively. The relation between their wavelengths i.e.,\[{{\lambda }_{1}}\] and \[{{\lambda }_{2}}\]will be
A)
\[{{\lambda }_{1}}=2{{\lambda }_{2}}\]
done
clear
B)
\[{{\lambda }_{1}}=4{{\lambda }_{2}}\]
done
clear
C)
\[{{\lambda }_{1}}=\frac{1}{2}{{\lambda }_{2}}\]
done
clear
D)
\[{{\lambda }_{1}}={{\lambda }_{2}}\]
done
clear
View Answer play_arrow
question_answer 96) Which of the following has the minimum bond length?
A)
\[\text{O}_{2}^{-}\]
done
clear
B)
\[\text{O}_{2}^{2-}\]
done
clear
C)
\[\text{O}_{2}^{{}}\]
done
clear
D)
\[\text{O}_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 97) Acidified \[{{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] solution turns green when \[\text{N}{{\text{a}}_{\text{2}}}\text{S}{{\text{O}}_{\text{3}}}\]is added to it. This is due to the formation of
A)
\[\text{CrO}_{4}^{2-}\]
done
clear
B)
\[\text{C}{{\text{r}}_{\text{2}}}{{\text{(S}{{\text{O}}_{\text{3}}}\text{)}}_{\text{3}}}\]
done
clear
C)
\[\text{CrS}{{\text{O}}_{4}}\]
done
clear
D)
\[\text{C}{{\text{r}}_{\text{2}}}{{\text{(S}{{\text{O}}_{4}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Which one of the following statements is not true?
A)
Concentration of DO below 6 ppm is good for the growth of fish
done
clear
B)
Clean water would have a BOD value of less than 5 ppm
done
clear
C)
Oxides of sulphur, nitrogen and carbon are the most widespread air pollutant
done
clear
D)
pH of drinking water should be between 5.5-9.5
done
clear
View Answer play_arrow
question_answer 99) If n = 6, the correct sequence for filling of electrons will be
A)
\[ns\xrightarrow[{}]{{}}(n-1)d\xrightarrow[{}]{{}}(n-2)f\xrightarrow[{}]{{}}np\]
done
clear
B)
\[ns-(n-2)f\xrightarrow[{}]{{}}np\xrightarrow[{}]{{}}(n-1)d\]
done
clear
C)
\[ns-np\xrightarrow[{}]{{}}(n-1)d\xrightarrow[{}]{{}}(n-2)f\]
done
clear
D)
\[ns\xrightarrow[{}]{{}}(n-2)f\xrightarrow[{}]{{}}(n-1)d\xrightarrow[{}]{{}}np\]
done
clear
View Answer play_arrow
question_answer 100) Which one of the following is present as an active ingredient in bleaching powder for bleaching action?
A)
\[\text{Ca(OCl}{{\text{)}}_{\text{2}}}\]
done
clear
B)
\[\text{Ca}{{\text{O}}_{\text{2}}}\text{Cl}\]
done
clear
C)
\[\text{CaC}{{\text{l}}_{\text{2}}}\]
done
clear
D)
\[\text{CaOC}{{\text{l}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 101) Given below is a sample of portion of DNA strand giving the base sequence on, the opposite strands. What is so, special shown in it? 5__GAATTC__3' 3'__CTTAAG__5'
A)
Deletion mutation
done
clear
B)
Start codon at the 5' end
done
clear
C)
Palindromic sequence of base pairs
done
clear
D)
Replication completed
done
clear
View Answer play_arrow
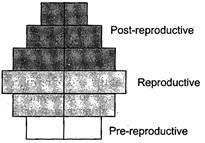
question_answer 102) Compared with the gametophytes of the bryophytes, the gametophytes of vascular plants tends to be
A)
larger but to have smaller sex organs
done
clear
B)
larger and to have large sex organs
done
clear
C)
smaller and to have smaller sex organs
done
clear
D)
smaller but to have larger sex organs
done
clear
View Answer play_arrow
question_answer 103) Which one of the following statements is correct?
A)
Seeds of orchids have oil-rich endosperm
done
clear
B)
Placentation in primrose is basal
done
clear
C)
Flower of tulip is a modified shoot
done
clear
D)
In tomato, fruit is a capsule
done
clear
View Answer play_arrow
question_answer 104) Arteries are best defined as the vessels which
A)
carry blood away from the heart to different organs
done
clear
B)
break up into capillaries which reunite to form a vein
done
clear
C)
carry blood from one visceral organ to another visceral organs
done
clear
D)
supply oxygenated blood to the different organs
done
clear
View Answer play_arrow
question_answer 105) There is a restriction endonuclease called Eco RI. What does 'co' part in it stand for?
A)
Coelom
done
clear
B)
Coenzyme
done
clear
C)
coli
done
clear
D)
Colon
done
clear
View Answer play_arrow
question_answer 106) Consider the following four conditions (I-IV) and select the correct pair of them as adaptation to environment in desert lizards. The conditions: I. Burrowing in soil to escape high temperature II. Losing heat rapidly from the body during high temperature III. Bask in sun when temperature is low IV. Insulating body due to thick fatty dermis
A)
(I) and (III)
done
clear
B)
(II) and (IV)
done
clear
C)
(I) and (II)
done
clear
D)
(III) and (IV)
done
clear
View Answer play_arrow
question_answer 107) 'Jaya' and 'Ratna' developed for green revolution in India are the varieties of
A)
rice
done
clear
B)
wheat
done
clear
C)
bajra
done
clear
D)
maize
done
clear
View Answer play_arrow
question_answer 108) Agarose extracted from sea weeds finds use in
A)
tissue culture
done
clear
B)
PCR
done
clear
C)
gel electrophoresis
done
clear
D)
spectrophotomeny
done
clear
View Answer play_arrow
question_answer 109)
Which one of the following structural formulae of two organic compounds is correctly identified along with its related function? -
A)
A ? Triglycerid ? source of energy
done
clear
B)
B ? Uracil ? a component of DNA
done
clear
C)
A ? Lecithin ? a component of cell membrane
done
clear
D)
B ? Adenine ? a nucleoride that makes up nucleic acids
done
clear
View Answer play_arrow
question_answer 110) A certain patient is suspected to be suffering from acquired immuno deficiency syndrome. Which diagnostic technique will you recommend for its detection?
A)
MRI
done
clear
B)
Ultra Sound
done
clear
C)
WIDAL
done
clear
D)
ELISA
done
clear
View Answer play_arrow
question_answer 111) Which one of the following statements is correct for secondary succession?
A)
It occurs on a deforested site
done
clear
B)
It follows primary succession
done
clear
C)
It is similar to primary succession except that it has a relatively fast pace
done
clear
D)
It begins on a bare rock
done
clear
View Answer play_arrow
question_answer 112) Filiform apparatus is a characteristic feature of
A)
egg
done
clear
B)
synergid
done
clear
C)
zygote
done
clear
D)
suspensor
done
clear
View Answer play_arrow
question_answer 113) Which one of the following pairs of gases are the major cause of 'Green house effect'?
A)
\[C{{O}_{2}}\] and CO
done
clear
B)
CFCs and \[S{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\] and \[{{N}_{2}}O\]
done
clear
D)
\[C{{O}_{2}}\] and \[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 114) Which one of the following expanded forms of the following acronyms is correct?
A)
UNEP ? United Nations Environmental Policy
done
clear
B)
EPA ? Environmental Pollution Agency
done
clear
C)
IUCN ? International Union for Conservation of Nature and Natural Resources
done
clear
D)
IPCC ? International Panel for Climate Change
done
clear
View Answer play_arrow
question_answer 115) What are those structures that appear, as 'beads-on-string' in the chromosomes when viewed under electron microscope?
A)
Nucleotides
done
clear
B)
Nucleosomes
done
clear
C)
Base pairs
done
clear
D)
Genes
done
clear
View Answer play_arrow
question_answer 116) Which one of the following statements for pyramid of energy is incorrect, whereas the remaining three are correct?
A)
It shows energy content of different trophic level organisms
done
clear
B)
It is inverted in shape
done
clear
C)
It is upright in shape
done
clear
D)
Its base is broad
done
clear
View Answer play_arrow
question_answer 117) Match the source gland with its.. respective hormone as well as the function,
A)
Source gland Hormone Function Posterior pituitary Vasopressin Stimulates re sorption of water in the distal tubules in the nephron
done
clear
B)
Corpus Iuteum Oestrogen Supports pregnancy
done
clear
C)
Thyroid Thyroxine Regulates
done
clear
D)
Anterior pituitary Oxytocin Contraction of uterus muscles during child birth
done
clear
View Answer play_arrow
question_answer 118) Medical Termination of Pregnancy (MTP) is considered safe up to have many weeks of pregnancy?
A)
Twelve weeks
done
clear
B)
Eighteen weeks
done
clear
C)
Six weeks
done
clear
D)
Eight weeks
done
clear
View Answer play_arrow
question_answer 119) The gametophyte is not an independent, free living generation in
A)
Adiantum
done
clear
B)
Marchantia
done
clear
C)
Pinus
done
clear
D)
Polytrichum
done
clear
View Answer play_arrow
question_answer 120) Which one of the following 'have the ?highest number of species in nature?
A)
Insects
done
clear
B)
Birds
done
clear
C)
Angiosperms
done
clear
D)
Fungi
done
clear
View Answer play_arrow
question_answer 121) Which one of the following statements is correct regarding blood pressure?
A)
100/55 mmHg is considered an ideal blood pressure
done
clear
B)
105/50 mmHg makes one very active
done
clear
C)
190/110 mmHg may harm vital organs like brain and kidney
done
clear
D)
130/90 mmHg is considered high and requires treatment
done
clear
View Answer play_arrow
question_answer 122)
Given ahead is an incomplete table about certain hormones, their source glands and one major effect of each on the body in humans. Identify the correct option for the three blanks A, B and C. Gland Secretion Effect on Body A Oestrogen Maintenance of second ary sexual characters Alpha cells of islets of Langerhans B Raises blood sugar level Anterior pituitary C Over secrection leads to gigantism
Options:
A)
A B C Placenta Insulin Vasopressin
done
clear
B)
Ovary Insulin Calcitonin
done
clear
C)
Placenta Glucagon Calcitonin
done
clear
D)
Ovary Glucagon Growth hormone
done
clear
View Answer play_arrow
question_answer 123) One very special feature in the earthworm Pheretima is that
A)
the typhlosole greatly increases the effective absorption area of the digested food in the intestine
done
clear
B)
the S-shaped setae embedded in the integument are the defensive weapons used against the enemies
done
clear
C)
it has a long dorsal tubular heart
done
clear
D)
fertilization of eggs occurs inside the body
done
clear
View Answer play_arrow
question_answer 124) The 'Eyes' of the potato tuber are
A)
flower buds
done
clear
B)
shoot buds
done
clear
C)
axillary buds
done
clear
D)
root buds
done
clear
View Answer play_arrow
question_answer 125) Which of the following is mainly produced by the activity of anaerobic bacteria on sewage?
A)
Propane
done
clear
B)
Mustard gas
done
clear
C)
Marsh gas
done
clear
D)
Laughing gas
done
clear
View Answer play_arrow
question_answer 126) Two friends are eating together on a dining table. One of them suddenly starts coughing while swallowing some food. This coughing would have been due to improper movement of
A)
diaphragm
done
clear
B)
neck
done
clear
C)
tongue
done
clear
D)
epiglottis
done
clear
View Answer play_arrow
question_answer 127)
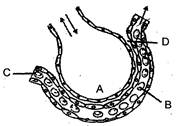
The figure given below shows a small part of human lung where exchange of gas takes place. In which one of the options given below, the one part A, B C or D is correctly identified along with its function.
A)
A - Alveolar cavity - main site of exchange of respiratory gases
done
clear
B)
D - Capillary wall - exchange of and takes place here
done
clear
C)
B - Red blood cell - transport of mainly
done
clear
D)
- Arterial capillary - passes oxygen to tissues
done
clear
View Answer play_arrow
question_answer 128) If for some reason, the vasa efferentia in the human reproductive system get blocked, the gametes will not be transported from
A)
epididymis to vas deferens
done
clear
B)
ovary to uterus
done
clear
C)
vagina to uterus
done
clear
D)
testes to epididymis
done
clear
View Answer play_arrow
question_answer 129) Which one of the following helps in absorption of phosphorus from soil by plants?
A)
Rhizobium
done
clear
B)
Frankia
done
clear
C)
Anabaena
done
clear
D)
Glomus
done
clear
View Answer play_arrow
question_answer 130) Large woody vines are more commonly found in
A)
mangroves
done
clear
B)
tropical rainforests
done
clear
C)
alpine forests
done
clear
D)
temperate forests
done
clear
View Answer play_arrow
question_answer 131) The ciliated columnar epithelial cells in humans are known to occur in
A)
bronchioles and fallopian tubes
done
clear
B)
bile duct and oesophagus
done
clear
C)
fallopian tubes and urethra
done
clear
D)
eustachian tube and stomach lining
done
clear
View Answer play_arrow
question_answer 132) A person with unknown blood group under ABO system, has suffered much blood loss in an accident and needs immediate blood transfusion. His one friend who- has a valid certificate of his own blood type, offers for blood donation without delay. What would have been the type of blood group of the donor friend?
A)
Type AB
done
clear
B)
Type O
done
clear
C)
Type A
done
clear
D)
Type B
done
clear
View Answer play_arrow
question_answer 133) Which one of the following-is the most widely accepted method of contraception in India as at 'present?
A)
Tubectbmy
done
clear
B)
Diaphragms
done
clear
C)
lUDs (Intra Uterine Devices)
done
clear
D)
Cervical caps
done
clear
View Answer play_arrow
question_answer 134) CAM helps the plants in
A)
secondary growth
done
clear
B)
disease resistance
done
clear
C)
reproduction
done
clear
D)
conserving water
done
clear
View Answer play_arrow
question_answer 135) The function of leghaemoglobin in the root nodules of legumes is
A)
oxygen removal
done
clear
B)
nodule differentiation
done
clear
C)
expression of nif gene
done
clear
D)
inhibition of nitrogenase activity
done
clear
View Answer play_arrow
question_answer 136) 'Bundle of His' is a part of which one of the following organs in humans?
A)
Heart
done
clear
B)
Kidney
done
clear
C)
Pancreas
done
clear
D)
Brain
done
clear
View Answer play_arrow
question_answer 137) Organisms called Methanogens are most abundant in a
A)
cattle yard
done
clear
B)
polluted stream
done
clear
C)
hot spring
done
clear
D)
sulphur rock
done
clear
View Answer play_arrow
question_answer 138) Which of the following enzymes carries out the initial step in the digestion of milk in humans?
A)
Rennin
done
clear
B)
Lipase
done
clear
C)
Trypsin
done
clear
D)
Pepsin
done
clear
View Answer play_arrow
question_answer 139) The purplish red pigment rhodopsin contained in the rods type of photoreceptor cells of the human eyes is a derivative of
A)
vitamin-C
done
clear
B)
vitamin-D
done
clear
C)
vitamin-A
done
clear
D)
vitamin-B
done
clear
View Answer play_arrow
question_answer 140) The process of RNA interference has been used in the development of plants resistant to
A)
fungi
done
clear
B)
viruses
done
clear
C)
insects
done
clear
D)
nematodes
done
clear
View Answer play_arrow
question_answer 141) Which one of the following organisms is not an example of eukaryotic cells?
A)
Escherichia coli
done
clear
B)
Euglena viridis
done
clear
C)
Amoeba, proteus
done
clear
D)
Paramecium caudatum
done
clear
View Answer play_arrow
question_answer 142) Which one of the following conditions correctly describes the manner of determining the sex in the given example?
A)
XO type of sex chromosomes determine male sex in grasshopper.
done
clear
B)
XO condition in humans as found in Turner syndrome, determines female sex
done
clear
C)
Homozygous sex chromosomes (XX) produce male in Drosophila
done
clear
D)
Homozygous sex chromosomes (ZZ) determine female sex in birds
done
clear
View Answer play_arrow
question_answer 143) Maximum number of existing transgenic animals is of
A)
mice
done
clear
B)
cow
done
clear
C)
pig
done
clear
D)
fish
done
clear
View Answer play_arrow
question_answer 144) Which one of the following shows maximum genetic diversity in India?
A)
Rice
done
clear
B)
Maize
done
clear
C)
Mango
done
clear
D)
Groundnut
done
clear
View Answer play_arrow
question_answer 145) Continuous addition of sugars in 'fed batch' fermentation is done to
A)
obtain antibiotics
done
clear
B)
purify enzymes
done
clear
C)
degrade sewage
done
clear
D)
produce methane
done
clear
View Answer play_arrow
question_answer 146) What would be the number of chromosomes of the aleurone cells of a plant with 42 chromosomes in its root tip cells?
A)
63
done
clear
B)
84
done
clear
C)
21
done
clear
D)
42
done
clear
View Answer play_arrow
question_answer 147) Nitrifying bacteria
A)
convert free nitrogen to nitrogen compounds
done
clear
B)
convert proteins into ammonia
done
clear
C)
reduce nitrates to free nitrogen
done
clear
D)
oxidize ammonia to nitrates
done
clear
View Answer play_arrow
question_answer 148) Secondary sewage treatment is mainly a
A)
mechanical process
done
clear
B)
chemical process
done
clear
C)
biological process
done
clear
D)
physical process
done
clear
View Answer play_arrow
question_answer 149) Which one of the following is not a bio fertilizer?
A)
Rhizobium
done
clear
B)
Nostoc
done
clear
C)
Mycorrhiza
done
clear
D)
Agrobacterium
done
clear
View Answer play_arrow
question_answer 150) Mass of living matter at a trophic level in an area at any time is called
A)
Detritus
done
clear
B)
Humus
done
clear
C)
Standing state
done
clear
D)
Standing crop
done
clear
View Answer play_arrow
question_answer 151) Which one of the following plasma proteins is involved in the coagulation of blood?
A)
Serum amylase
done
clear
B)
A globulin
done
clear
C)
Fibrinogen
done
clear
D)
An albumin
done
clear
View Answer play_arrow
question_answer 152)
What type of human population is represented by the following age pyramid?
A)
Stable population
done
clear
B)
Declining population
done
clear
C)
Expanding population
done
clear
D)
Vanishing population
done
clear
View Answer play_arrow
question_answer 153) Important site for formation of glycoproteins and glycolipids is
A)
Golgi apparatus
done
clear
B)
plastid
done
clear
C)
lysosome
done
clear
D)
vacuole
done
clear
View Answer play_arrow
question_answer 154) Which of the following is correctly states as it happens in the common cockroach?
A)
Oxygen is transported by haemoglobin in blood
done
clear
B)
Nitrogenous excretory product is urea
done
clear
C)
The food is ground by mandibles and gizzard
done
clear
D)
Malpighian tubules are excretory organs projecting out from the colon
done
clear
View Answer play_arrow
question_answer 155) Which one of the following correctly explains the function of a specific part of a human nephron?
A)
Henle's loop ? most reabsoroption of the major substances from the glomerular filtrate
done
clear
B)
Distal convoluted? reabsorprtion of ions into tubule the surrounding blood capillaries
done
clear
C)
Afferent arteriole ? carries the blood away from the glomerulus towards renal vein
done
clear
D)
Podocytes ? create minute spaces (slit pores) for the filtration of blood into the Bowman's capsule
done
clear
View Answer play_arrow
question_answer 156) What will you look for to identity the sex of the following?
A)
Male frog ? a copulatory pad on the first digit of the hind limb
done
clear
B)
Female cock- ? anal cerci roach
done
clear
C)
Male shark ? claspers borne on pelvic fins
done
clear
D)
Female Ascaris ? sharply curved posterior end
done
clear
View Answer play_arrow
question_answer 157) Select the correct option with respect to mitosis.
A)
Chromatids start moving towards opposite poles in telophase
done
clear
B)
Golgi complex and endoplasmic reticulum are still visible at the end of prophase
done
clear
C)
Chromosomes move to the spindle equator and get aligned along equatorial plate in metaphase
done
clear
D)
Chromatids separate but remains in the centre of the cell in anaphase
done
clear
View Answer play_arrow
question_answer 158) A collection of plants and seeds having diverse alleles of all the genes of a crop is called
A)
germplasm
done
clear
B)
gene library
done
clear
C)
genome
done
clear
D)
herbarium
done
clear
View Answer play_arrow
question_answer 159) The correct floral formula of chilli is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 160) The most common substrate used in distilleries for the production of ethanol is
A)
soya meal
done
clear
B)
ground gram
done
clear
C)
molasses
done
clear
D)
corn meal
done
clear
View Answer play_arrow
question_answer 161) Of the total incident solar radiation the proportion of PAR is
A)
about 60%
done
clear
B)
less than 50%
done
clear
C)
more than 80%
done
clear
D)
about 70%
done
clear
View Answer play_arrow
question_answer 162) Which one of the following elements in plants is not remobilised?
A)
Calcium
done
clear
B)
Potassium
done
clear
C)
Sulphur
done
clear
D)
Phosphorus
done
clear
View Answer play_arrow
question_answer 163) Ethanol is commercially produced through a particular species of
A)
Clostridium
done
clear
B)
Trichoderma
done
clear
C)
Asperyllus
done
clear
D)
Saccharomyces
done
clear
View Answer play_arrow
question_answer 164) A large proportion of oxygen is left unsused in the human blood even after its uptake by the body tissues. This \[{{O}_{2}}\]
A)
raises the \[{{p}_{C{{O}_{2}}}}\]of blood to 75 mm of Hg
done
clear
B)
is enough to keep pxyhaemoglobin
done
clear
C)
helps in relasing more \[{{O}_{2}}\] to the epithelial tissues
done
clear
D)
acts as a reserve during muscular exercise
done
clear
View Answer play_arrow
question_answer 165) Which one of the following is categorised as a parasite in true sense?
A)
Human foetus developing inside the uterus draws nourishment from the mother
done
clear
B)
Head louse living on the human scalp as well as laying eggs on human hair
done
clear
C)
The cuckoo (koel) lays its eggs in crow's nest
done
clear
D)
The female Anopheles bites and sucks blood from humans
done
clear
View Answer play_arrow
question_answer 166) At which stage of HIV infection does one usually shows symptoms of AIDS?
A)
When viral DNA is produced by reverse transcriptase
done
clear
B)
When HIV replicates rapidly in helper T-lymphocytes and damages large number of these
done
clear
C)
With 15 days of sexual contact with an infected person
done
clear
D)
When the infecting retrovirus enters host cells
done
clear
View Answer play_arrow
question_answer 167) Which one of the following statements is wrong in case Bhopal gas tragedy?
A)
Thousands of human beings died
done
clear
B)
Radioactive fallout engulfed Bhopal
done
clear
C)
It took place in the night of December 2/3, 1984
done
clear
D)
Methyl isocyanate gas leakage took place
done
clear
View Answer play_arrow
question_answer 168) Ground tissue includes
A)
all tissues except epidermis and vascular bundles
done
clear
B)
epidermis and cortex
done
clear
C)
all tissues internal to endodermis
done
clear
D)
all tissuses external to endodermis
done
clear
View Answer play_arrow
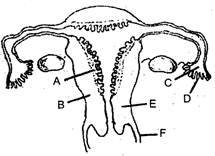
question_answer 169)
The figure given below depicts a diagrammatic sectional view of the female reproductive system of humans. Which one set of three parts out of A-F have been correctly identified?
A)
C-Infundibulum, D-Fimbriae, E-Cervix
done
clear
B)
D-Oviducal runnel, E-Uterus, F-Cervix
done
clear
C)
A-Perimeo-ium, B-Myometrium, C-Pallopian tube
done
clear
D)
B-Endometrium, C-Infundibulum, D-Fimbriae
done
clear
View Answer play_arrow
question_answer 170) Eutrophication is often seen in
A)
fresh water lakes
done
clear
B)
ocean
done
clear
C)
mountains
done
clear
D)
deserts
done
clear
View Answer play_arrow
question_answer 171) Flowers are zygomorphic in
A)
gulmohur
done
clear
B)
tomato
done
clear
C)
datura
done
clear
D)
mustard
done
clear
View Answer play_arrow
question_answer 172) A drupe develope in
A)
wheat
done
clear
B)
pea
done
clear
C)
tomato
done
clear
D)
mango,
done
clear
View Answer play_arrow
question_answer 173) Which one of the following also acts as a catalyst in a bacterial cell?
A)
sn RNA
done
clear
B)
hn RNA
done
clear
C)
23 S rRNA
done
clear
D)
5 S Rrna
done
clear
View Answer play_arrow
question_answer 174) What was the most significant trend in the evolution of modem man (Homo sapiens) from his ancestors?
A)
Shortening of jaws
done
clear
B)
Binocular vision
done
clear
C)
Increasing brain capacity
done
clear
D)
Upright posture
done
clear
View Answer play_arrow
question_answer 175) In eubacteria, a cellular component that resembles eukaryotic cells is
A)
nucleus
done
clear
B)
ribosomes
done
clear
C)
cell wall
done
clear
D)
plasma membrane
done
clear
View Answer play_arrow
question_answer 176) In which one of the following the genus name, its two characters and its class/phylum are correctly matched?
A)
Genus Two characters Class/phylum Salaman dra (i) A tympanum represents hair Amphibia
done
clear
B)
Pteropus (i) Skin possesses hair Mammalia
done
clear
C)
(ii) Oviparous Aurelia (i) Cnidoblas Coelenterata (ii) Organ level of organization
done
clear
D)
Ascaris (i) Body segmented Annelida (ii) Males and Females distinct
done
clear
View Answer play_arrow
question_answer 177) Which one of the following animals is correctly mathed with its particular named taxonomic category?
A)
Cuttlefish ? Mollusca, a class
done
clear
B)
Humans ? Primata, the family
done
clear
C)
Housefly ? Musca, an order
done
clear
D)
Tiger ? tigris, the species
done
clear
View Answer play_arrow
question_answer 178) Mutations can be induced with
A)
IAA
done
clear
B)
ethylene
done
clear
C)
gamma radiations
done
clear
D)
infra red radiations
done
clear
View Answer play_arrow
question_answer 179) In land plants, the guard cells differ from other epidermal cells in having
A)
mitochondria
done
clear
B)
enddplasmic reticulum
done
clear
C)
chloroplasts
done
clear
D)
cytoskeleton
done
clear
View Answer play_arrow
question_answer 180) Wind pollination is common in
A)
lilies
done
clear
B)
grasses
done
clear
C)
orchids
done
clear
D)
legumes
done
clear
View Answer play_arrow
question_answer 181) Which one of the following is not a part of a renal pyramid?
A)
Convoluted tubules
done
clear
B)
Collecting ducts
done
clear
C)
Loops Henie
done
clear
D)
Peritubular capillaries
done
clear
View Answer play_arrow
question_answer 182) 'Himgiri' developed by hybridisation and selection for disease resistance against rust pathogens is a variety of
A)
maize
done
clear
B)
sugarcane
done
clear
C)
wheat
done
clear
D)
chilli
done
clear
View Answer play_arrow
question_answer 183) Which one of the following groups of animals is" correctly matched with its one characteristic feature without even a single exception?
A)
Chordata ?possess a mouth provided with an upper and a lower Jaw
done
clear
B)
Chondrichthyes?possess cartilaginous endoskeleton
done
clear
C)
Mammalia ? give birth to young ones
done
clear
D)
Reptilia ?possess 3-chambered heart with one incompletely divided ventricle
done
clear
View Answer play_arrow
question_answer 184) Which one of the following acts as a physiological barrier to the entry of microorganisms in human body?
A)
Tears
done
clear
B)
Monocytes
done
clear
C)
Skin
done
clear
D)
Epithelium of urogenital tract
done
clear
View Answer play_arrow
question_answer 185) When a neuron is an resting state, ie, not conducting any impulse, the axonal membrane is
A)
equally permeable to both \[N{{a}^{+}}\] and \[{{K}^{+}}\] ions
done
clear
B)
impermeable to both \[N{{a}^{+}}\] and \[{{K}^{+}}\] ions
done
clear
C)
comparatively more permeable to \[{{K}^{+}}\] ions and nearly impermeable to \[N{{a}^{+}}\] ions
done
clear
D)
comparatively more permeable to \[N{{a}^{+}}\] ions and nearly impermeable to \[{{K}^{+}}\]ions
done
clear
View Answer play_arrow
question_answer 186) Nucellar polyembryony is reported in species of
A)
Gossypium
done
clear
B)
Triticum
done
clear
C)
Brassica
done
clear
D)
Citrus
done
clear
View Answer play_arrow
question_answer 187) Peptide synthesis inside a cell takes place in
A)
mitochondria
done
clear
B)
chromoplast
done
clear
C)
ribosomes
done
clear
D)
chloroplast
done
clear
View Answer play_arrow
question_answer 188) When two unrelated individuals or lines are crossed, the performance of \[{{F}_{1}}\] hybrid is often superior to, both its parents. This phenomenon is called
A)
transformation
done
clear
B)
splicing
done
clear
C)
metamorphosis
done
clear
D)
heterosis
done
clear
View Answer play_arrow
question_answer 189) The cork cambium, cork and secondary cortex are collectively called
A)
phellogen
done
clear
B)
periderm
done
clear
C)
phellem
done
clear
D)
phelloderm
done
clear
View Answer play_arrow
question_answer 190)
The curve given below shows enzymatic activity with relation to three conditions (pH, temperature and substrate concentration) What do the two axises (X and Y) represent?
A)
X-axis Y-axis temperature ? enzyme activity
done
clear
B)
substrate concentration ? enzymatic activity
done
clear
C)
enzymatic activity ? temperature
done
clear
D)
enzymatic activity ? pH
done
clear
View Answer play_arrow
question_answer 191) Uricotelic mode of passing out nitrogenous wastes is found in
A)
birds and annelids
done
clear
B)
amphibians and reptiles
done
clear
C)
insects and amphibians
done
clear
D)
reptiles and birds
done
clear
View Answer play_arrow
question_answer 192) The testes in humans are situated outside the abdominal cavity inside a pouch called scrotum. The purpose served is for
A)
escaping any possible compression by the visceral organs
done
clear
B)
providing more space for/the growth of epididymis
done
clear
C)
providing a secondary sexual feature for exhibiting the male sex
done
clear
D)
maintaining the scrotal temperature lower than the internal body temperature
done
clear
View Answer play_arrow
question_answer 193) An organism used as a biofertilizer for raising soyabean crop is
A)
Azospirillum
done
clear
B)
Rhizobium
done
clear
C)
Nostoc
done
clear
D)
Azotobacter
done
clear
View Answer play_arrow
question_answer 194) Which one of the following is wrongly matched?
A)
Puccinia ? Smut
done
clear
B)
Root ? Exarch protoxylem
done
clear
C)
Cassia ? Imbricate aestivation
done
clear
D)
Root pressure ? Guttation
done
clear
View Answer play_arrow
question_answer 195) In which one of the following pollination is autogamous?
A)
Xenogamy
done
clear
B)
Chasmogamy
done
clear
C)
Cleistogamy
done
clear
D)
Geitonogamy
done
clear
View Answer play_arrow
question_answer 196) The ovary is half inferior in flowers of
A)
cucumber
done
clear
B)
cotton
done
clear
C)
guava
done
clear
D)
peach
done
clear
View Answer play_arrow
question_answer 197) Which one of the following statements is correct with respect to kidney function regulation?
A)
Exposure to cold temperature stimulates ADH release
done
clear
B)
An increase in glomerular blood flow stimulates formation of angiotensin II
done
clear
C)
During summer when body loses lot of water by evaporation, the release of ADH is suppressed
done
clear
D)
When someone drinks lot of water ADH release is supposed
done
clear
View Answer play_arrow
question_answer 198) Archegoniophore is present in
A)
Chara
done
clear
B)
Adiantum
done
clear
C)
Furiaria
done
clear
D)
Marchantia
done
clear
View Answer play_arrow
question_answer 199) Where will you look for the sporozoites of the malarial parasite?
A)
Red blood corpuscles of humans suffering from malaria
done
clear
B)
Spleen of infected humans
done
clear
C)
Salivary glands of freshly moulted female Anopheles mosquito
done
clear
D)
Saliva of infected female Anopheles mosquito
done
clear
View Answer play_arrow
question_answer 200) A prokaryofic autotrophic nitrogen fixing symbiont is found in
A)
Cycas
done
clear
B)
Cicer
done
clear
C)
Pisum
done
clear
D)
Ainus
done
clear
View Answer play_arrow