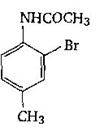
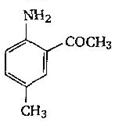
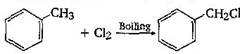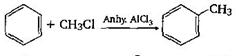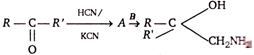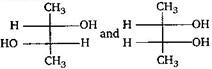-
question_answer1) A man weighs 80 kg. He stands on a weighing scale in a lift which is moving upwards with a uniform acceleration of \[5\,m/{{s}^{2}}\]. What would be the reading on the scale? \[(g=10\,\,m/{{s}^{2}})\]
A)
800 N
done
clear
B)
1200 N
done
clear
C)
Zero
done
clear
D)
400 N
done
clear
View Answer play_arrow
-
question_answer2) A monkey of mass 20 kg is holding a vertical rope. The rope will not break when a mass of 25 kg is suspended from it but will break if the mass exceeds 25 kg. What is the maximum acceleration with which the monkey can climb up along the rope? \[(g=10\,\,m/{{s}^{2}})\]
A)
\[25\,\,m/{{s}^{2}}\]
done
clear
B)
\[2.5\,\,m/{{s}^{2}}\]
done
clear
C)
\[5\,\,m/{{s}^{2}}\]
done
clear
D)
\[10\,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer3) J.J. Thomson's cathode-ray tube experiment demonstrated that:
A)
the e/m of electrons is greater than the e/m of protons
done
clear
B)
the e/m ratio of the cathode-ray particles changes when a different gas is placed in the discharge tube
done
clear
C)
cathode rays axe streams of negatively charged ions
done
clear
D)
all the mass of an atom is essentially in the nucleus
done
clear
View Answer play_arrow
-
question_answer4) Reverse bias applied to a junction diode:
A)
increases the majority carrier current
done
clear
B)
increases the minority carrier current
done
clear
C)
lowers the potential barrier
done
clear
D)
raises the potential barrier
done
clear
View Answer play_arrow
-
question_answer5) In which of the following systems will the radius of the first orbit (n = 1) be minimum?
A)
Deuterium atom
done
clear
B)
Hydrogen atom
done
clear
C)
Doubly ionized lithium
done
clear
D)
Singly ionized helium
done
clear
View Answer play_arrow
-
question_answer6) A bar magnet is oscillating in the earth's magnetic field with a period T. What happens to its period and motion if its mass is quadrupled?
A)
Motion remains SH with time period = 4T
done
clear
B)
Motion remains SH and period remains nearly constant
done
clear
C)
Motion remains SH with time period = T/2
done
clear
D)
Motion remains SH with time period = 2T
done
clear
View Answer play_arrow
-
question_answer7) A charged particle moves through a magnetic field in a direction perpendicular to it. Then the:
A)
acceleration remains unchanged
done
clear
B)
velocity remains unchanged
done
clear
C)
speed of the particle remains unchanged
done
clear
D)
direction of the particle remains unchanged
done
clear
View Answer play_arrow
-
question_answer8) A thin circular ring of mass M and radius r is rotating about its axis with a constant angular velocity \[\omega \]. Four objects each of mass m, are kept gently to the opposite ends of two perpendicular diameters of the ring. The angular velocity of the ring will be:
A)
\[\frac{(M+4m)\,\omega }{M}\]
done
clear
B)
\[\frac{(M-4m)\,\omega }{M+4m}\]
done
clear
C)
\[\frac{M\,\omega }{4m}\]
done
clear
D)
\[\frac{M\,\omega }{M+4m}\]
done
clear
View Answer play_arrow
-
question_answer9) A long solenoid carrying a current produces a magnetic field B along its axis. If the current is doubled and the number of rums per cm is halved, the new value of the magnetic field is
A)
2B
done
clear
B)
4B
done
clear
C)
B/2
done
clear
D)
B
done
clear
View Answer play_arrow
-
question_answer10)
Following diagram performs the logic function of: 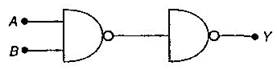
A)
OR gate
done
clear
B)
AND gate
done
clear
C)
XOR gate
done
clear
D)
NAND gate
done
clear
View Answer play_arrow
-
question_answer11) The volume occupied by an atom is greater than the volume of the nucleus by factor of about:
A)
\[{{10}^{10}}\]
done
clear
B)
\[{{10}^{15}}\]
done
clear
C)
\[{{10}^{1}}\]
done
clear
D)
\[{{10}^{5}}\]
done
clear
View Answer play_arrow
-
question_answer12) The mass number of a nucleus is:
A)
sometimes equal to its atomic number
done
clear
B)
sometimes less than and sometimes more than its atomic number
done
clear
C)
always less than its atomic number
done
clear
D)
always more than its atomic number
done
clear
View Answer play_arrow
-
question_answer13) The mass of proton is 1.0073 u and that of neutron is 1.0087 u (u = atomic mass unit) The binding energy of \[_{2}H{{e}^{4}}\] is:
A)
28.4 MeV
done
clear
B)
0.061 u
done
clear
C)
0.0305 J
done
clear
D)
0.0305 erg
done
clear
View Answer play_arrow
-
question_answer14) Barrier potential of a p-n junction diode does not depend on:
A)
forward bias
done
clear
B)
doping density
done
clear
C)
diode design
done
clear
D)
Temperature
done
clear
View Answer play_arrow
-
question_answer15) A particle moves along a circle of radius \[\left( \frac{20}{\pi } \right)\] m with constant tangential acceleration. If the velocity of the particle is 80 m/s at the end of the second revolution after motion has begun, the tangential acceleration is:
A)
\[160\,\pi \,m/{{s}^{2}}\]
done
clear
B)
\[40\,\,m/{{s}^{2}}\]
done
clear
C)
\[40\,\pi \,m/{{s}^{2}}\]
done
clear
D)
\[640\,\pi \,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer16) An electric kettle has two heating coils. When one of the coils is connected to an AC source, the water in the kettle boils in 10 min. When the other coil is used the water boils in 40 min. If both the coils are connected in parallel, the time taken by the same quantity of water to boil will be:
A)
25 min
done
clear
B)
15 min
done
clear
C)
8 min
done
clear
D)
4 min
done
clear
View Answer play_arrow
-
question_answer17) If a ball is thrown vertically upwards with speed u, the distance covered during the last t seconds of its ascent is:
A)
\[ut-\frac{1}{2}g\,{{t}^{2}}\]
done
clear
B)
(u + gt) t
done
clear
C)
ut
done
clear
D)
\[\frac{1}{2}g{{t}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer18) Two 220 V, 100 W bulbs are connected first in series and then in parallel. Each time the combination is connected to a 220 V AC supply line. The power drawn by the combination in each case respectively will be:
A)
200 W, 150 W
done
clear
B)
50 W, 200 W
done
clear
C)
50 W, 100 W
done
clear
D)
100 W, 50 W
done
clear
View Answer play_arrow
-
question_answer19) Fuse wire is a wire of:
A)
low resistance and low melting point
done
clear
B)
low resistance and high melting point
done
clear
C)
high resistance and high melting point
done
clear
D)
high resistance and low melting point
done
clear
View Answer play_arrow
-
question_answer20) Solar energy is mainly caused due to:
A)
fusion of protons during synthesis of heavier elements
done
clear
B)
gravitational contraction
done
clear
C)
burning of hydrogen in the oxygen
done
clear
D)
fission of uranium present in the sun
done
clear
View Answer play_arrow
-
question_answer21)
An equiconvex lens is cut into two halves along (i) XOX' and (ii) YOY' as shown in the figure. Let \[f,\,f',\,f''\] be the focal lengths of the complete lens, of each half in case (i), and of each half in case (ii), respectively. 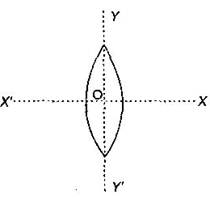 Choose the correct statement from the following:
Choose the correct statement from the following:
A)
\[f'=f,\,\,f''=f\]
done
clear
B)
\[f'=2f,\,\,f''=2f\]
done
clear
C)
\[f'=f,\,\,f''=2f\]
done
clear
D)
\[f'=2f,\,\,f''=f\]
done
clear
View Answer play_arrow
-
question_answer22) A nuclear reaction given by: \[_{Z}{{X}^{A}}{{\to }_{Z+1}}{{Y}^{A}}{{+}_{-1}}{{e}^{0}}+\overline{v}\] represents:
A)
fusion
done
clear
B)
fission
done
clear
C)
\[\beta \]-decay
done
clear
D)
\[\gamma \]-decay
done
clear
View Answer play_arrow
-
question_answer23) A sample of radioactive element has a mass of 10 g at an instant t = 0. The approximate mass of this element in the sample after two mean lives is:
A)
3.70 g
done
clear
B)
6.30 g
done
clear
C)
1.35 g
done
clear
D)
2.50 g
done
clear
View Answer play_arrow
-
question_answer24) A photoelectric cell is illuminated by a point source of light 1 m away. When the source is shifted to 2 m then:
A)
each emitted electron carries half the initial energy
done
clear
B)
number of electrons emitted is a quarter of the initial number
done
clear
C)
each emitted electron carries one quarter of the initial energy
done
clear
D)
number of electrons emitted is half the initial number
done
clear
View Answer play_arrow
-
question_answer25) The vector sum of two forces is perpendicular to their vector differences. In that case, the forces:
A)
are not equal to each other in magnitude
done
clear
B)
cannot be predicted
done
clear
C)
are equal to each other
done
clear
D)
are equal to each other in magnitude
done
clear
View Answer play_arrow
-
question_answer26) A ball rolls without slipping. The radius of gyration of the ball about an axis passing through its centre of mass is K. If radius of the ball be R, then the fraction of total energy associated with its rotational energy will be:
A)
\[\frac{{{K}^{2}}}{{{K}^{2}}+{{R}^{2}}}\]
done
clear
B)
\[\frac{{{R}^{2}}}{{{K}^{2}}+{{R}^{2}}}\]
done
clear
C)
\[\frac{{{K}^{2}}+{{R}^{2}}}{{{R}^{2}}}\]
done
clear
D)
\[\frac{{{K}^{2}}}{{{R}^{2}}}\]
done
clear
View Answer play_arrow
-
question_answer27) The acceleration due to gravity on the planet A is 9 times the acceleration due to gravity on planet B. A man jumps to a height of 2m on the surface of A. What is the height of jump by the same person on the planet B?
A)
6 m
done
clear
B)
\[\frac{2}{3}\] m
done
clear
C)
\[\frac{2}{9}\] m
done
clear
D)
18 m
done
clear
View Answer play_arrow
-
question_answer28) When a long spring is stretched by 2 cm, its potential energy is U. If the spring is stretched by 10 cm, the potential energy in it will be:
A)
10 U
done
clear
B)
25 U
done
clear
C)
U/5
done
clear
D)
5 U
done
clear
View Answer play_arrow
-
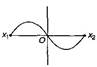
question_answer29) A particle of mass m oscillates with simple harmonic motion between points \[{{x}_{1}}\] and \[{{x}_{2}}\] the equilibrium position being O. Its potential energy is plotted. It will be as given below in the graph:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer30) An electron is moving round the nucleus of a hydrogen atom in a circular orbit of radius r. The coulomb force \[\vec{F}\] between the two is:
A)
\[k\frac{{{e}^{2}}}{{{r}^{3}}}\vec{r}\]
done
clear
B)
\[-k\,\frac{{{e}^{2}}}{{{r}^{3}}}\vec{r}\]
done
clear
C)
\[k\frac{{{e}^{2}}}{{{r}^{2}}}\hat{r}\]
done
clear
D)
\[-k\frac{{{e}^{2}}}{{{r}^{3}}}\hat{r}\]
done
clear
View Answer play_arrow
-
question_answer31) A charge q is located at the centre of a cube. The electric flux through any face is:
A)
\[\frac{\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
B)
\[\frac{q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
C)
\[\frac{2\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
D)
\[\frac{4\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
View Answer play_arrow
-
question_answer32) An observer moves towards a stationary source of sound with a speed 1/5th of the speed of sound. The wavelength and frequency of the source emitted are \[\lambda \] and f respectively. The apparent frequency and wavelength recorded by the observer are respectively:
A)
\[f,\,1,\,2\lambda \]
done
clear
B)
\[0.8f,\,0.8\lambda \]
done
clear
C)
\[1.2f,\,1.2\lambda \]
done
clear
D)
\[1.2\,f,\,\lambda \]
done
clear
View Answer play_arrow
-
question_answer33) Motion of observer does not affect the wavelength reaching the observer, hence, wavelength remains \[\lambda \]. Consider a compound slab consisting of two different materials having equal thickneses and thermal conductivities K and 2K, respectively. The equivalent thermal conductivity of the slab is:
A)
3K
done
clear
B)
\[\frac{4}{3}K\]
done
clear
C)
\[\frac{2}{3}K\]
done
clear
D)
\[\sqrt{2}\,K\]
done
clear
View Answer play_arrow
-
question_answer34) If a full wave rectifier circuit is operating from 50 Hz mains, the fundamental frequency in the ripple will be:
A)
70.7 Hz
done
clear
B)
100 Hz
done
clear
C)
25 Hz
done
clear
D)
59 Hz
done
clear
View Answer play_arrow
-
question_answer35) A diamagnetic material in a magnetic field moves:
A)
perpendicular to the field
done
clear
B)
from weaker to the stronger parts of the field
done
clear
C)
from stronger to the weaker parts of the field
done
clear
D)
in none of the above directions
done
clear
View Answer play_arrow
-
question_answer36) A convex lens is dipped in a liquid whose refractive index is equal to the refractive index of the lens. Then its focal length will:
A)
become small, but non-zero
done
clear
B)
remain unchanged
done
clear
C)
become zero
done
clear
D)
become infinite
done
clear
View Answer play_arrow
-
question_answer37) A n-p-n transistor conducts when:
A)
collector is positive and emitter is at same potential as the base
done
clear
B)
both collector and emitter are negative with respect to the base
done
clear
C)
both collector and emitter are positive with respect to the base
done
clear
D)
collector is positive and emitter is negative with respect to the base
done
clear
View Answer play_arrow
-
question_answer38) Which of the following rays are not electromagnetic waves?
A)
\[\beta \]-rays
done
clear
B)
Heat rays
done
clear
C)
X-rays
done
clear
D)
\[\gamma \]-rays
done
clear
View Answer play_arrow
-
question_answer39) A man throws balls with the same speed vertically upwards one after the other at an interval of 2 seconds. What should be the speed of the throw so that more than two balls are in the sky at any time? (Given \[g\]\[=9.8\,m/{{s}^{2}}\])
A)
Any speed less than 19.6 m/s
done
clear
B)
Only with speed 19.6 m/s
done
clear
C)
More than 19.6 m/s
done
clear
D)
At least 9.8 m/s
done
clear
View Answer play_arrow
-
question_answer40) Two spheres of masses \[m\] and \[M\] are situated in air and the gravitational force between them is \[F\]. The space around the masses is now filled with a liquid of specific gravity 3. The gravitational force will now be:
A)
\[\frac{F}{3}\]
done
clear
B)
\[\frac{F}{9}\]
done
clear
C)
3F
done
clear
D)
F
done
clear
View Answer play_arrow
-
question_answer41) The potential energy of a simple harmonic oscillator when the particle is half way to its end point is:
A)
\[\frac{1}{4}F\]
done
clear
B)
\[\frac{1}{2}F\]
done
clear
C)
\[\frac{2}{3}F\]
done
clear
D)
\[\frac{1}{8}F\] (where E is the total energy)
done
clear
View Answer play_arrow
-
question_answer42) According to Curie's law, the magnetic susceptibility of a paramagnetic substance at an absolute temperature T is proportional to:
A)
\[\frac{1}{{{T}^{2}}}\]
done
clear
B)
\[{{T}^{2}}\]
done
clear
C)
\[\frac{1}{T}\]
done
clear
D)
T
done
clear
View Answer play_arrow
-
question_answer43) The time period of a mass suspended from a spring is T. If the spring is cut into four equal parts and the same mass is suspended from one of the parts, then the new time period will be:
A)
\[\frac{T}{2}\]
done
clear
B)
2T
done
clear
C)
\[\frac{T}{4}\]
done
clear
D)
T
done
clear
View Answer play_arrow
-
question_answer44) In case of a forced vibration, the resonance wave becomes very sharp when the:
A)
applied periodic force is small
done
clear
B)
quality factor is small
done
clear
C)
damping force is small
done
clear
D)
restoring force is small
done
clear
View Answer play_arrow
-
question_answer45) A solid cylinder of mass M and radius R rolls without slipping down an inclined plane of length L and height h What is the speed of its centre of mass when the cylinder reaches its bottom?
A)
\[\sqrt{\frac{4}{3}gh}\]
done
clear
B)
\[\sqrt{4gh}\]
done
clear
C)
\[\sqrt{2gh}\]
done
clear
D)
\[\sqrt{\frac{3}{4}gh}\]
done
clear
View Answer play_arrow
-
question_answer46) A stationary particle explodes into two particles of masses \[{{m}_{1}}\] and \[{{m}_{2}}\] which move in opposite directions with velocities \[{{v}_{1}}\] and \[{{v}_{2}}\]. The ratio of their kinetic energies \[{{E}_{1}}/{{E}_{2}}\] is:
A)
1
done
clear
B)
\[{{m}_{1}}{{v}_{2}}/{{m}_{2}}{{v}_{1}}\]
done
clear
C)
\[{{m}_{2}}/{{m}_{1}}\]
done
clear
D)
\[{{m}_{1}}/{{m}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer47) An ideal gas heat engine operates in a Carnot cycle between \[{{227}^{o}}C\] and \[{{127}^{o}}C\]. It absorbs 6 kcal at the higher temperature. The amount of heat (in kcal) converted into work is equal to:
A)
1.6
done
clear
B)
1.2
done
clear
C)
4.8
done
clear
D)
3.5
done
clear
View Answer play_arrow
-
question_answer48) We consider the radiation emitted by the human body. Which of the following statements is true?
A)
The radiation is emitted during the summers and absorbed during the winters
done
clear
B)
The radiation emitted lies in the ultraviolet region and hence is not visible
done
clear
C)
The radiation emitted is in the infra-red region
done
clear
D)
The radiation is emitted only during the day
done
clear
View Answer play_arrow
-
question_answer49) Three capacitors each of capacity \[4\,\mu F\] are to be connected in such a way that the effective capacitance is \[6\,\mu F\]. This can be done by:
A)
connecting two in series and one in parallel
done
clear
B)
connecting two in parallel and one in series
done
clear
C)
connecting all of diem in series
done
clear
D)
connecting all of them in parallel
done
clear
View Answer play_arrow
-
question_answer50) In a Wheatstone's bridge all the four arms have equal resistance R. If the resistance of the galvanometer arm is also R, the equivalent resistance of the combination as seen by the battery is:
A)
R
done
clear
B)
2R
done
clear
C)
\[\frac{R}{4}\]
done
clear
D)
\[\frac{R}{2}\]
done
clear
View Answer play_arrow
-
question_answer51) The correct order of reactivity towards the electrophilic substitution of the compounds aniline (I), benzene (II) and nitrobenzene (III) is:
A)
II < III > I
done
clear
B)
I > II > III
done
clear
C)
III > II > I
done
clear
D)
II > III > I
done
clear
View Answer play_arrow
-
question_answer52) The densities of graphite and diamond at 298 K are 2.25 and \[3.31\,\,g\,\,c{{m}^{-3}},\] respectively. If the standard free energy difference (\[\Delta \]\[{{G}^{\text{o}}}\]) is equal to 1895 J mol-1, the pressure at which graphite will be transformed into diamond at 298 K is:
A)
\[9.92\times {{10}^{6}}\,Pa\]
done
clear
B)
\[9.92\times {{10}^{5}}\,Pa\]
done
clear
C)
\[9.92\times {{10}^{8}}\,Pa\]
done
clear
D)
\[9.92\times {{10}^{7}}Pa\]
done
clear
View Answer play_arrow
-
question_answer53) Which one of the following is a free radical substitution reaction?
A)
done
clear
B)
\[C{{H}_{3}}CHO+HCN\xrightarrow[{}]{{}}C{{H}_{3}}CH(OH)CN\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer54) The radioisotope, tritium \[(\,_{1}^{3}H)\] has a half-life of 12.3 years. If the initial amount of tritium is 32 mg, how many milligrams of it would remain after 49.2 years?
A)
4 mg
done
clear
B)
8 mg
done
clear
C)
1 mg
done
clear
D)
2 mg
done
clear
View Answer play_arrow
-
question_answer55) The reaction A\[\to \]B follows first order kinetics. The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 hour. What is the time taken for conversion of 0.9 mole of A to produce 0.675 mole of B?
A)
0.25 h
done
clear
B)
2 h
done
clear
C)
1 h
done
clear
D)
0.5 h
done
clear
View Answer play_arrow
-
question_answer56) On the basis of the information available from the reaction \[\frac{4}{3}Al+{{O}_{2}}\to \frac{2}{3}\,A{{l}_{2}}{{O}_{3}},\,\Delta G=-827\,kJ\,mo{{l}^{-1}}\,of\]\[{{O}_{2}}\], the minimum emf required to carry out an electrolysis of \[A{{l}_{2}}{{O}_{3}}\] is \[(F=96500\,\,C\,mo{{l}^{-1}})\]
A)
6.42 V
done
clear
B)
8.56 V
done
clear
C)
2.14 V
done
clear
D)
4.28 V
done
clear
View Answer play_arrow
-
question_answer57)
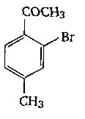
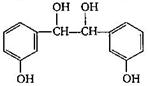
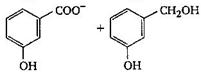
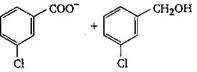
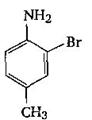
The final product C, obtained in this reaction 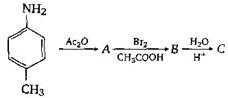
A)
would be:
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer58) In this reaction: \[\begin{align} & C{{H}_{3}}CHO+HCN\xrightarrow[{}]{{}}C{{H}_{3}}CH(OH)CN \\ & \xrightarrow{H.OH}C{{H}_{3}}CH(OH)COOH \\ \end{align}\] an asymmetric centre is generated. The acid obtained would be:
A)
50% D + 50% L-isomer
done
clear
B)
20% D + 80% L-isomer
done
clear
C)
D-isomer
done
clear
D)
L-isomer
done
clear
View Answer play_arrow
-
question_answer59)
Name of the compound given below is: 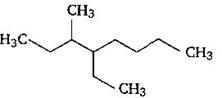
A)
2, 3-diethylheptane
done
clear
B)
5-ethyl-6-methyloctane
done
clear
C)
4-ethyl-3-methyloctane
done
clear
D)
3-methyl-4-ethyloctane
done
clear
View Answer play_arrow
-
question_answer60) According to the adsorption theory of catalysis, the speed of the reaction increase because:
A)
absorption produces heat which increases the speed of the reaction
done
clear
B)
adsorption lowers the activation energy of the reaction
done
clear
C)
the concentration of reactant molecules at the active centres of the catalyst becomes high due to adsorption
done
clear
D)
in the process of adsorption, the activation energy of the molecules becomes large
done
clear
View Answer play_arrow
-
question_answer61) The emf of a Daniell cell at 298 K is \[{{E}_{1}}\] \[ZN\,\left| \begin{align} & ZnS{{O}_{4}} \\ & (0.01\,M) \\ \end{align} \right|\,\left| \begin{align} & CuS{{O}_{4}} \\ & (1.0\,M) \\ \end{align} \right|Cu\] When the concentration of \[ZnS{{O}_{4}}\] is 1.0M and that of \[CuS{{O}_{4}}\] is 0.01 M, the emf changed to \[{{E}_{2}}\]. What is the relationship between \[{{E}_{1}}\] and \[{{E}_{2}}\]?
A)
\[{{E}_{1}}={{E}_{2}}\]
done
clear
B)
\[{{E}_{2}}=0\,\ne {{E}_{1}}\]
done
clear
C)
\[{{E}_{1}}>E{{ & }_{2}}\]
done
clear
D)
\[{{E}_{1}}<{{E}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer62) Vitamin \[{{B}_{12}}\] contains:
A)
Zn (II)
done
clear
B)
Ca (II)
done
clear
C)
Fe (II)
done
clear
D)
Co (III)
done
clear
View Answer play_arrow
-
question_answer63) Which one of the following octahedral complexes will not show geometrical isomerism? (A and B are monodentate ligands)
A)
\[[M{{A}_{4}}{{B}_{2}}]\]
done
clear
B)
\[[M{{A}_{5}}B]\]
done
clear
C)
\[[M{{A}_{2}}{{B}_{4}}]\]
done
clear
D)
\[[M{{A}_{3}}{{B}_{3}}]\]
done
clear
View Answer play_arrow
-
question_answer64) Acrilan is a hard, horny and a high melting material. Which of the following represents its structure?
A)
\[{{\left( \begin{align} & -C{{H}_{2}}-\underset{|}{\mathop{C}}\,H- \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,COO{{C}_{2}}{{H}_{5}} \\ \end{align} \right)}_{n}}\,\,\]
done
clear
B)
\[{{\left( \begin{align} & -C{{H}_{2}}-\underset{|}{\mathop{C}}\,H- \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Cl \\ \end{align} \right)}_{n}}\]
done
clear
C)
\[{{\left( \begin{align} & C{{H}_{2}}-\underset{|}{\mathop{C}}\,H- \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,CN \\ \end{align} \right)}_{n}}\]
done
clear
D)
\[{{\left( \begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}} \\ & -C{{H}_{2}}-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,- \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,COOC{{H}_{3}} \\ \end{align} \right)}_{n}}\]
done
clear
View Answer play_arrow
-
question_answer65) The molar heat capacity of water at constant pressure, C, is \[75\,\,J{{K}^{-1}}\,mo{{l}^{-1}},\] When 1.0 kJ of heat is supplied to 100 g of water which is free to expand, the increase in temperature of water is :
A)
4.8 K
done
clear
B)
6.6 K
done
clear
C)
1.2 K
done
clear
D)
2.4 K
done
clear
View Answer play_arrow
-
question_answer66) The reaction quotient (Q) for the reaction \[{{N}_{2}}(g)\,+3{{H}_{2}}(g)\] \[\rightleftharpoons \] \[2N{{H}_{3}}(g)\] is given by \[Q=\frac{{{[N{{H}_{3}}]}^{2}}}{[{{N}_{2}}]\,{{[{{H}_{2}}]}^{3}}}\] . The reaction will proceed towards right side if:
A)
\[Q>{{K}_{c}}\]
done
clear
B)
Q = 0
done
clear
C)
\[Q={{K}_{c}}\]
done
clear
D)
\[Q<{{K}_{c}}\]
done
clear
View Answer play_arrow
-
question_answer67) What is the entropy change (in \[J{{K}^{-1}}\,mo{{l}^{-1}}\]) when one mole of ice is converted into water at \[{{0}^{\text{o}}}C\]? (The enthalpy change for the conversion of ice to liquid water is \[6.0\,kJ\,mo{{l}^{-1}}\] at \[{{0}^{\text{o}}}C\].)
A)
2.198
done
clear
B)
21.98
done
clear
C)
20.13
done
clear
D)
2.013
done
clear
View Answer play_arrow
-
question_answer68) The method of zone refining of metals is based on the principle of:
A)
greater noble character of the solid metal than that of the impurity
done
clear
B)
greater solubility of the impurity in the molten state than in the solid
done
clear
C)
greater mobility of the pure metal than that of impurity
done
clear
D)
higher melting point of the impurity than that of the pure metal
done
clear
View Answer play_arrow
-
question_answer69) According to IUPAC nomenclature sodium nitroprusside is named as:
A)
sodium pentacyanonitrosyl ferrate (II)
done
clear
B)
sodium pentacyanonitrosyl ferrate (III)
done
clear
C)
sodium nitroferricyanide
done
clear
D)
sodium nitroferrocyanide
done
clear
View Answer play_arrow
-
question_answer70) Phospholipids are esters of glycerol with:
A)
one carboxylic acid residue and two phosphate groups
done
clear
B)
three phoshate groups
done
clear
C)
three carboxylic acid residues
done
clear
D)
two carboxylic acid residues and one phosphate groups
done
clear
View Answer play_arrow
-
question_answer71) The oxidation states of sulphur in the anions \[SO_{3}^{2-},\,{{S}_{2}}O_{4}^{2-}\,and\,{{S}_{2}}O_{6}^{2-}\] follow the order:
A)
\[{{S}_{2}}O_{4}^{2-}<{{S}_{2}}O_{6}^{2-}<SO_{3}^{2-}\]
done
clear
B)
\[{{S}_{2}}O_{6}^{2-}<{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}\]
done
clear
C)
\[{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}<{{S}_{2}}O_{6}^{2-}\]
done
clear
D)
\[SO_{3}^{2-}<{{S}_{2}}O_{4}^{2-}<{{S}_{2}}O_{6}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer72) The value of Planck's constant is \[6.63\times {{10}^{34}}Js\]. The velocity of light is \[3.0\times {{10}^{8}}m{{s}^{-1}}\]. Which value is closest to the wavelength in nanometers of a quantum of light with frequency of \[8\times {{10}^{15}}{{s}^{-1}}\]?
A)
\[4\times {{10}^{1}}\]
done
clear
B)
\[3\times {{10}^{7}}\]
done
clear
C)
\[2\times {{10}^{-25}}\]
done
clear
D)
\[5\times {{10}^{-18}}\]
done
clear
View Answer play_arrow
-
question_answer73) The ions \[{{O}^{2-}},\,{{F}^{-}},\,N{{a}^{+}},M{{g}^{2+}}\,and\,\,A{{l}^{3+}}\]are isoelectronic. Their ionic radii show:
A)
an increase from \[{{O}^{2-}}\] to F- and then decrease from \[N{{a}^{+}}\] to \[A{{l}^{3+}}\]
done
clear
B)
a decrease from \[{{O}^{2-}}\] to F- and then increase from \[N{{a}^{+}}\] to \[A{{l}^{3+}}\]
done
clear
C)
a significant increase from \[{{O}^{2-}}\] to \[A{{l}^{3+}}\]
done
clear
D)
a significant decrease from \[{{O}^{2-}}\] to \[A{{l}^{3+}}\]
done
clear
View Answer play_arrow
-
question_answer74) The temperature dependence of rate constant (k) of a chemical reaction is written in terms of Arrhenius equation, \[k=A{{e}^{-E*/RT}}\]. Activation energy \[({{E}^{*}})\] of the reaction can be calculated by ploting:
A)
\[\log \,k\,vs\frac{1}{T}\]
done
clear
B)
\[\log \,k\,vs\,\frac{1}{\log \,T}\]
done
clear
C)
k vs T
done
clear
D)
\[k\,vs\frac{1}{\log \,T}\]
done
clear
View Answer play_arrow
-
question_answer75) If the rate of the reaction is equal to the rate constant, the order of the reaction is:
A)
2
done
clear
B)
3
done
clear
C)
0
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer76) Which one of the following monomers gives the polymer neoprene on polymerization?
A)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Cl} \\ & \text{C}{{\text{H}}_{\text{2}}}\text{ = }\overset{\text{ }\!\!|\!\!\text{ }}{\mathop{\text{C}}}\,-CH=C{{H}_{2}} \\ \end{align}\]
done
clear
B)
\[C{{F}_{2}}=C{{F}_{2}}\]
done
clear
C)
\[C{{H}_{2}}=CHCl\]
done
clear
D)
\[CC{{l}_{2}}=CC{{l}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer77)
A and B in the following reactions are 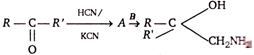
A)
\[A=RR'C{{H}_{2}}CN,\,B=NaOH\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer78) Glycolysis is:
A)
oxidation of glucose to pyruvate
done
clear
B)
conversion of glucose to haem
done
clear
C)
oxidation of glucose to glutamate
done
clear
D)
conversion of pyruvate to citrate
done
clear
View Answer play_arrow
-
question_answer79) The basic character 6f the transition metal monoxides follows the order:
A)
TiO > FeO > VO > CrO
done
clear
B)
TiO > VO > CrO > FeO
done
clear
C)
VO > CrO > TiO > FeO
done
clear
D)
CrO > VO > FeO > TiO
done
clear
View Answer play_arrow
-
question_answer80) Which of the following statements is not true?
A)
HOCl is a stronger add than HOBr
done
clear
B)
HF is a stronger acid than HCl
done
clear
C)
Among halide tons, iodide is the most powerful reducing agent
done
clear
D)
Fluorine is the only halogen that does not show a variable oxidation state
done
clear
View Answer play_arrow
-
question_answer81) The compound \[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}} \\ & C{{H}_{3}}-\overset{|}{\mathop{C}}\,=CH-C{{H}_{3}} \\ \end{align}\] on reaction with \[NaC{{O}_{4}}\] hi the presence of \[KMn{{O}_{4}}\] gives:
A)
\[C{{H}_{3}}COC{{H}_{3}}+C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{3}}CHO+C{{O}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}+C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
-
question_answer82) In a set of the given reactions, acetic acid yielded a product C, \[C{{H}_{3}}COOH+PC{{l}_{5}}\to A\xrightarrow[Anh.\,AlC{{l}_{3}}]{{{C}_{6}}{{H}_{6}}}B\underset{Ether}{\mathop{\xrightarrow{{{C}_{2}}{{H}_{5}}MgBr}}}\,C\] product C would be:
A)
\[C{{H}_{3}}CH(OH){{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} {{C}_{2}}{{H}_{5}} \\ | \end{smallmatrix}}{\mathop{C(OH){{C}_{6}}{{H}_{5}}}}\,\]
done
clear
C)
\[C{{H}_{3}}CH(OH){{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[C{{H}_{3}}CO{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer83) Which of the following pairs of compounds are enantiomers?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer84) In Haber process 30 L of dihydrogen and 30 L of dinitrogen were taken for reaction which yielded only 50% of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end?
A)
20L ammonia, 10L nitrogen, 30L hydrogen
done
clear
B)
20L ammonia, 25L nitrogen, 15L hydrogen
done
clear
C)
20L ammonia, 20L nitrogen, 20L hydrogen
done
clear
D)
10L ammonia, 25L nitrogen, 15L hydrogen
done
clear
View Answer play_arrow
-
question_answer85) Which one of the following orders of acid strength is correct?
A)
RCOOH > HOH > HC \[\equiv \] CH > ROH
done
clear
B)
RCOOH >HC \[\equiv \] CH > HOH > ROH
done
clear
C)
RCOOH > ROH > HOH > HC \[\equiv \] CH
done
clear
D)
RCOOH > HOH > ROH > HC \[\equiv \] CH
done
clear
View Answer play_arrow
-
question_answer86) When m-chlorobenzaldehyde is treated with 50% KOH solution, the product(s) obtained is (are):
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer87) The following equilibria are given: \[\begin{matrix} {{N}_{2}}+3{{H}_{2}}2N{{H}_{3}} & {{K}_{1}} \\ \end{matrix}\] \[\begin{matrix} {{N}_{2}}+{{O}_{2}}2NO & {{K}_{2}} \\ \end{matrix}\] \[\begin{matrix} {{H}_{2}}+\frac{1}{2}{{O}_{2}}{{H}_{2}}O & {{K}_{3}} \\ \end{matrix}\] The equilibrium constant of the reaction \[2N{{H}_{3}}+\frac{5}{2}{{O}_{2}}\rightleftharpoons 2NO+3{{H}_{2}}O\] in terms of \[{{K}_{1}},\,{{K}_{2}}\] and \[{{K}_{3}}\] is:
A)
\[\frac{{{K}_{1}}K_{3}^{2}}{{{K}_{2}}}\]
done
clear
B)
\[\frac{{{K}_{2}}\,K_{3}^{3}}{{{K}_{1}}}\]
done
clear
C)
\[{{K}_{1}}\,{{K}_{2}}\,{{K}_{3}}\]
done
clear
D)
\[\frac{{{K}_{1}}\,{{K}_{2}}}{{{K}_{3}}}\]
done
clear
View Answer play_arrow
-
question_answer88) For which one of the following equations \[\Delta H_{nreact}^{o}\] equal to \[\Delta H_{f}^{o}\] for the product?
A)
\[Xe(g)+2{{F}_{2}}(g)\xrightarrow[{}]{{}}Xe{{F}_{4}}(g)\]
done
clear
B)
\[2CO(g)+{{O}_{2}}(g)\xrightarrow[{}]{{}}2C{{O}_{2}}(g)\]
done
clear
C)
\[{{N}_{2}}(g)+{{O}_{3}}(g)\xrightarrow[{}]{{}}2C{{O}_{2}}(g)\]
done
clear
D)
\[C{{H}_{4}}(g)+2C{{l}_{2}}(g)\xrightarrow[{}]{{}}C{{H}_{2}}C{{l}_{2}}(\ell )+2HCl\,(g)\]
done
clear
View Answer play_arrow
-
question_answer89) Which of the following statements is not correct for sigma- and pi-bonds formed between two carbon atoms?
A)
Free rotation of atoms about a sigma-bond is allowed but not in case of a pi-bond
done
clear
B)
Sigma-bond determines the direction between carbon atoms but a pi-bond has no primary effect in this regard
done
clear
C)
Sigma-bond is stronger than a pi-bond
done
clear
D)
Bond energies of sigma- and pi-bonds are of the order of 264 kJ/mol and 347 kJ/mol, respectively
done
clear
View Answer play_arrow
-
question_answer90) Which one of the following compounds is not a protonic acid?
A)
\[SO{{(OH)}_{2}}\]
done
clear
B)
\[S{{O}_{2}}{{(OH)}_{2}}\]
done
clear
C)
* \[B{{(OH)}_{3}}\]
done
clear
D)
\[PO{{(OH)}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer91) Among the following, which is not the \[\pi \]-bonded organometallic compound?
A)
\[Cr{{({{\eta }^{6}}-{{C}_{6}}{{H}_{6}})}_{2}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{4}}Sn\]
done
clear
C)
\[K[PtC{{l}_{3}}\,({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})]\]
done
clear
D)
\[Fe{{({{\eta }^{5}}-{{C}_{5}}{{H}_{5}})}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer92) The correct order of ionic radii of \[{{Y}^{3+}},\,L{{a}^{3+}},\,E{{u}^{3+}}\] and \[L{{u}^{3+}}\] is:
A)
\[L{{u}^{3+}}<E{{u}^{3+}}<L{{a}^{3+}}<{{Y}^{3+}}\]
done
clear
B)
\[L{{a}^{3+}}<E{{u}^{3+}}<L{{u}^{3+}}<{{Y}^{3+}}\]
done
clear
C)
\[{{Y}^{3+}}<L{{a}^{3+}}<E{{u}^{3+}}<L{{u}^{3+}}\]
done
clear
D)
\[{{Y}^{3+}}<L{{u}^{3+}}<E{{u}^{3+}}<L{{a}^{3+}}\]
done
clear
View Answer play_arrow
-
question_answer93) Which one of the following characteristics of the transition metals is associated with their catalytic activity?
A)
Colour of hydrated ions
done
clear
B)
Variable oxidation states
done
clear
C)
High enthalpy of atomization
done
clear
D)
Paramagnetic behavior
done
clear
View Answer play_arrow
-
question_answer94) The number of unpaired electrons in me complex ion \[{{[Co{{F}_{6}}]}^{3-}}\] is:
A)
4
done
clear
B)
zero
done
clear
C)
2
done
clear
D)
3 (Atomic number Co = 27)
done
clear
View Answer play_arrow
-
question_answer95) Chargaffs rule states that in an organism:
A)
amount of adenine (A) is equal to that of cytosine (C) and die amount of thymine (T) is equal to that of guanine (G)
done
clear
B)
amounts of all bases are equal
done
clear
C)
amount of adenine (A) is equal to that of thymine (T) and the amount of guanine (G) is equal to that of cytosine (C)
done
clear
D)
amount of adenine (A) is equal to that of guanine (G) and the amount of thymine (T) is equal to that of cytosine (C)
done
clear
View Answer play_arrow
-
question_answer96) The activation energy for a simple chemical reaction A \[\to \] B is \[{{E}_{a}}\] in forward direction. The activation energy for reverse reaction:
A)
can be less than or more than \[{{E}_{a}}\]
done
clear
B)
is always double of \[{{E}_{a}}\]
done
clear
C)
is negative of \[{{E}_{a}}\]
done
clear
D)
is always less than \[{{E}_{a}}\]
done
clear
View Answer play_arrow
-
question_answer97) Formation of a solution from two components can be considered as: (1) pure solvent \[\to \] separated solvent molecules, \[\Delta {{H}_{1}}\] (2) pure solute \[\to \] separated solute molecules, \[\Delta {{H}_{2}}\] (3) separated solvent and solute molecules \[\to \] solution, \[\Delta {{H}_{3}}\] Solution so formed will be ideal if:
A)
\[\Delta {{H}_{so\ln }}=\Delta {{H}_{1}}-\Delta {{H}_{2}}-\Delta {{H}_{3}}\]
done
clear
B)
\[\Delta {{H}_{so\ln }}=\Delta {{H}_{3}}-\Delta {{H}_{1}}-\Delta {{H}_{2}}\]
done
clear
C)
\[\Delta {{H}_{so\ln }}=\Delta {{H}_{1}}+\Delta {{H}_{2}}+\Delta {{H}_{3}}\]
done
clear
D)
\[\Delta {{H}_{so\ln }}=\Delta {{H}_{1}}+\Delta {{H}_{2}}-\Delta {{H}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer98) The solubility product of Agl at \[{{25}^{\text{o}}}C\] is \[1.0\times {{10}^{-16}}\,mo{{l}^{2}}\,{{L}^{-2}}\]. The solubility of \[AgI\] in \[{{10}^{-4}}N\] solution of KI at \[{{25}^{\text{o}}}C\] is approximately (in mol \[{{L}^{-1}}\]):
A)
\[1.0\times {{10}^{-10}}\]
done
clear
B)
\[1.0\times {{10}^{-8}}\]
done
clear
C)
\[1.0\times {{10}^{-16}}\]
done
clear
D)
\[1.0\times {{10}^{-12}}\]
done
clear
View Answer play_arrow
-
question_answer99) For the reaction, \[{{C}_{3}}{{H}_{8}}(g)+5{{O}_{2}}(g)\xrightarrow[{}]{{}}3C{{O}_{2}}(g)+4{{H}_{2}}O\,(l)\] at constant temperature, \[\Delta H-\Delta E\] is
A)
+3RT
done
clear
B)
-RT
done
clear
C)
+RT
done
clear
D)
-3RT
done
clear
View Answer play_arrow
-
question_answer100) The pyknometric density of sodium chloride crystal is \[2.165\,\times {{10}^{3}}\,kg{{m}^{-3}}\] while its X-ray density is \[2.178\,\times {{10}^{3}}\,kg\,{{m}^{-3}}\]. The fraction of unoccupied sites in sodium chloride crystal is:
A)
\[5.96\,\times {{10}^{-1}}\]
done
clear
B)
\[5.96\,\times {{10}^{-3}}\]
done
clear
C)
5.96
done
clear
D)
\[5.96\,\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
-
question_answer101) Genetic map is one chat:
A)
shows the stages during the cell division
done
clear
B)
shows the distribution of various species in a region
done
clear
C)
establishes sites of the genes on a chromosome
done
clear
D)
establishes the various stages in gene evolution
done
clear
View Answer play_arrow
-
question_answer102) Coconut milk factor is:
A)
abscisic acid
done
clear
B)
cytokinin
done
clear
C)
an auxin
done
clear
D)
a gibberellins
done
clear
View Answer play_arrow
-
question_answer103) Which one of the following triplet codes, is correctly matched with its specificity for an amino acid in protein synthesis or as 'start' or 'stop' codon?
A)
UGU?Leucine
done
clear
B)
UAC?Tyrosine
done
clear
C)
UCG?Start
done
clear
D)
UUU?Stop
done
clear
View Answer play_arrow
-
question_answer104) Tobacco mosaic virus is a tubular filament of size:
A)
\[300\times 20\,\,nm\]
done
clear
B)
\[700\times 30\,\,nm\]
done
clear
C)
\[300\times 10\,\,nm\]
done
clear
D)
\[300\times 5\,\,nm\]
done
clear
View Answer play_arrow
-
question_answer105) ELISA is used to detect viruses where the key reagent is:
A)
DNA probe
done
clear
B)
RNAase
done
clear
C)
alkaline phosphatase
done
clear
D)
catalase
done
clear
View Answer play_arrow
-
question_answer106) Convergent evolution is illustrated by:
A)
starfish and cuttle fish
done
clear
B)
dogfish and whale
done
clear
C)
rat and dog
done
clear
D)
bacterium and protozoan
done
clear
View Answer play_arrow
-
question_answer107) During translation initiation in prokaryotes, a GTP molecule is needed in:
A)
association of 30S, m-RNA with formyl-met-t-RNA
done
clear
B)
association of 50S subunit of ribosome with initiation complex
done
clear
C)
formation of formyl-met-t-RNA
done
clear
D)
binding of 30S subunit of ribosome with m-RNA
done
clear
View Answer play_arrow
-
question_answer108) Sycon belongs to a group of animals, which are best described as:
A)
multicellular with a gastrovascular system
done
clear
B)
multicellular having tissue organization, but no body cavity
done
clear
C)
unicellular or acellular
done
clear
D)
multicellular without any tissue organization
done
clear
View Answer play_arrow
-
question_answer109) Viruses are no more ?alive? than isolated chromosomes because:
A)
they both require oxygen for respiration
done
clear
B)
both require the environment of a cell to replicate
done
clear
C)
they require both RNA and DNA
done
clear
D)
they both need food molecules
done
clear
View Answer play_arrow
-
question_answer110) Cellular totipotency is demonstrated by:
A)
all eukaryotic cells
done
clear
B)
only bacterial cells
done
clear
C)
only gymnosperm cells
done
clear
D)
all plant cells
done
clear
View Answer play_arrow
-
question_answer111) Which one of the following concerns photophosphorylation?
A)
ADP + Inorganic \[P{{O}_{4}}\] \[\to \] ATP
done
clear
B)
AMP + Inorganic \[P{{O}_{4}}\xrightarrow[{}]{Light\,energy}ATP\]
done
clear
C)
ADP + AMP \[\xrightarrow[{}]{Light\,energy}ATP\]
done
clear
D)
\[\text{ADP}+\text{Inorganic P}{{\text{O}}_{4}}\xrightarrow{\text{Light energy}}\text{ATP}\]
done
clear
View Answer play_arrow
-
question_answer112) What is true about T-lymphocytes in mammals?
A)
They scavenge damaged cells and cellular debris
done
clear
B)
These are produced in thyroid
done
clear
C)
There are three main types?cytotoxic T-cells, helper T-cells and suppressor T-cells
done
clear
D)
These originate in lymphoid tissues
done
clear
View Answer play_arrow
-
question_answer113) In recent years, DNA sequences (nucleotide sequence) of mt-DNA and Y-chromosomes were considered for the study of human evolution, because:
A)
their structure is known in greater detail
done
clear
B)
they can be studied from the samples of fossil remains
done
clear
C)
they are small and therefore, easy to study
done
clear
D)
they are uniparental in origin and do not take part in recombination
done
clear
View Answer play_arrow
-
question_answer114) Which one of the following is a matching pair of an animal and a certain phenomenon it exhibits?
A)
Chameleon ? Mimicry
done
clear
B)
Taenia ? Polymorphism
done
clear
C)
Pheretima ? Sexual dimorphism
done
clear
D)
Musca ? Complete metamorphosis
done
clear
View Answer play_arrow
-
question_answer115) Which fractions of the visible spectrum of solar radiations are primarily absorbed by carotenoids of the higher plants?
A)
Red and violet
done
clear
B)
Violet and blue
done
clear
C)
Blue and green
done
clear
D)
Green and red
done
clear
View Answer play_arrow
-
question_answer116) Species are considered as:
A)
artificial concept of human mind which cannot be defined in absolute terms
done
clear
B)
real units of classification devised by taxonomists
done
clear
C)
real basic units of classification
done
clear
D)
the lowest units of classification
done
clear
View Answer play_arrow
-
question_answer117) Random genetic drift in a population probably results from:
A)
constant low mutation rate
done
clear
B)
large population size
done
clear
C)
highly genetically variable individuals
done
clear
D)
interbreeding within this population
done
clear
View Answer play_arrow
-
question_answer118) If Henle's loop were absent from mammalian nephron, which of the following is to be expected?
A)
The urine will be more concentrated
done
clear
B)
The urine will be more dilute
done
clear
C)
There will be no urine formation
done
clear
D)
There will be hardly any change in the quality and quantity of urine formed
done
clear
View Answer play_arrow
-
question_answer119) During prolonged fasting, in what sequence are the following organic compounds used up by the body?
A)
First carbohydrates, next proteins and lastly lipids
done
clear
B)
First proteins, next lipids and lastly carbohydrate
done
clear
C)
First carbohydrates, next fats and lastly proteins
done
clear
D)
First fats, next carbohydrates and lastly proteins
done
clear
View Answer play_arrow
-
question_answer120) What used to be described as Nissl granules in a nerve cell are now identified as?
A)
Ribosomes
done
clear
B)
Mitochondria
done
clear
C)
Cell metabolites
done
clear
D)
Fat granules
done
clear
View Answer play_arrow
-
question_answer121) Genes for cytoplasmic male sterility in plants are generally located in:
A)
nuclear genome
done
clear
B)
cytosol
done
clear
C)
chloroplast genome
done
clear
D)
mitochondrial genome
done
clear
View Answer play_arrow
-
question_answer122) The apical meristem of the root is present:
A)
only in adventitious roots
done
clear
B)
in all the roots
done
clear
C)
only in radicals
done
clear
D)
only in tap roots
done
clear
View Answer play_arrow
-
question_answer123) Diffuse porous woods are characteristic of plants growing in:
A)
temperate climate
done
clear
B)
tropics
done
clear
C)
alpine region
done
clear
D)
cold winter regions
done
clear
View Answer play_arrow
-
question_answer124) In which one of the following nitrogen is not a constituent?
A)
Invertase
done
clear
B)
Pepsin
done
clear
C)
done
clear
D)
Bacteriochlorophyll
done
clear
View Answer play_arrow
-
question_answer125) Which one of the following is wrong in relation to photorespiration?
A)
It is a characteristics of \[{{C}_{4}}\] plants
done
clear
B)
It is a characteristics of \[{{C}_{3}}\] plants
done
clear
C)
It occurs in chloroplasts
done
clear
D)
It occurs in daytime only
done
clear
View Answer play_arrow
-
question_answer126) Stomata of a plant open due to:
A)
influx of hydrogen ions
done
clear
B)
influx of calcium ions
done
clear
C)
influx of potassium ions
done
clear
D)
efflux of potassium ions
done
clear
View Answer play_arrow
-
question_answer127) Stomata of CAM plants:
A)
open during the night and close during the day
done
clear
B)
never open
done
clear
C)
are always open
done
clear
D)
open during the day and close at night
done
clear
View Answer play_arrow
-
question_answer128) The major portion of the dry weight of plants comprises of:
A)
carbon, nitrogen and hydrogen
done
clear
B)
carbon, hydrogen and oxygen
done
clear
C)
nitrogen, phosphorus and potassium
done
clear
D)
calcium, magnesium and sulphur
done
clear
View Answer play_arrow
-
question_answer129) In Drosbphtia, the sex is determined by:
A)
the ratio of pairs of X-chromosomes to the pairs of autosomes
done
clear
B)
whether the egg is fertilized or develops parthenogenetically
done
clear
C)
the ratio of number of X-chromosomes to the set of autosomes
done
clear
D)
X and Y-chromosomes
done
clear
View Answer play_arrow
-
question_answer130) Christmas disease in another name for:
A)
Down's syndrome
done
clear
B)
sleeping sickness
done
clear
C)
haemophilia B
done
clear
D)
hepatitis B
done
clear
View Answer play_arrow
-
question_answer131) When a cluster of genes show linkage behaviour they:
A)
do not show independent assortment
done
clear
B)
induce cell division
done
clear
C)
do not show a chromosome map
done
clear
D)
show recombination during meiosis
done
clear
View Answer play_arrow
-
question_answer132) Degeneration of a genetic code is attributed to the:
A)
entire codon
done
clear
B)
third member of a codon
done
clear
C)
first member of a codon
done
clear
D)
second member of a codon
done
clear
View Answer play_arrow
-
question_answer133) Two opposite forces operate in the growth and development of every population. One of them relates to die ability to reproduce at a given rate. The force opposing it is called:
A)
biotic potential
done
clear
B)
environmental resistance
done
clear
C)
morbidity
done
clear
D)
fecundity
done
clear
View Answer play_arrow
-
question_answer134) Fluoride pollution mainly affects:
A)
teeth
done
clear
B)
kidney
done
clear
C)
brain
done
clear
D)
heart
done
clear
View Answer play_arrow
-
question_answer135) Which one of the following statements about viruses is correct?
A)
Viruses are obligate parasites
done
clear
B)
Nucleic acid of viruses is known as capsid
done
clear
C)
Viruses possess their own metabolic system
done
clear
D)
All viruses contain both RNA and DNA
done
clear
View Answer play_arrow
-
question_answer136) Sexual reproduction in Spirogyra is an advanced feature because it shows:
A)
morphologically different sex organs
done
clear
B)
physiologically differentiated sex organs
done
clear
C)
different sizes of motile sex organs
done
clear
D)
same size of motile sex organs
done
clear
View Answer play_arrow
-
question_answer137) Phenetic classification is based on:
A)
dendograms based on DNA characteristics
done
clear
B)
sexual characteristics
done
clear
C)
the ancestral lineage of existing organisms
done
clear
D)
observable characteristics of existing organisms
done
clear
View Answer play_arrow
-
question_answer138) In alcohol fermentation:
A)
there is no electron donor
done
clear
B)
oxygen is the electron acceptor
done
clear
C)
triose phosphate is the electron donor while acetaldehyde is the electron acceptor
done
clear
D)
triose phosphate is the electron donor while pyruvic acid is the electron acceptor
done
clear
View Answer play_arrow
-
question_answer139) During anaerobic digestion of organic waste, such as in producing biogas, which one of the following is left undegraded?
A)
Hemicellulose
done
clear
B)
Cellulose
done
clear
C)
Lipids
done
clear
D)
Lignin
done
clear
View Answer play_arrow
-
question_answer140) Industrial melanism is an example of:
A)
protective resemblance with the surroundings
done
clear
B)
defensive adaptation of skin against ultraviolet radiations
done
clear
C)
drug resistance
done
clear
D)
darkening of skin due to smoke from industries
done
clear
View Answer play_arrow
-
question_answer141) The cells of the quiescent centre are characterised by:
A)
dividing regularly to add to the corpus
done
clear
B)
dividing regularly to add to tunica
done
clear
C)
having dense cytoplasm and prominent nuclei
done
clear
D)
having light cytoplasm and small nuclei
done
clear
View Answer play_arrow
-
question_answer142) Differentiation of shoot is controlled by:
A)
high gibberellin : auxin ratio
done
clear
B)
high gibberellin : cytokinin ratio
done
clear
C)
high auxin : cytokinin ratio
done
clear
D)
high cytokinin : auxin ratio
done
clear
View Answer play_arrow
-
question_answer143) In a flowering plant, archesporium gives rise to:
A)
wall and die tapetum
done
clear
B)
only tapetum and sporogenous cells
done
clear
C)
only the wall of the sporangium
done
clear
D)
both wall and the sporogenous cells
done
clear
View Answer play_arrow
-
question_answer144) Which one of die following mineral elements plays an important role in biological nitrogen fixation?
A)
Zinc
done
clear
B)
Molybdenum
done
clear
C)
Copper
done
clear
D)
Manganese
done
clear
View Answer play_arrow
-
question_answer145) Plants deficient of element zinc, show its effect on the biosynthesis of plant growth hormone:
A)
ethylene
done
clear
B)
abscisic acid
done
clear
C)
auxin
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
-
question_answer146) In sugarcane plant \[^{14}C{{O}_{2}}\] is fixed in a malic acid, in which the enzyme that fixes \[C{{O}_{2}}\] is:
A)
ribulose phosphate kinase
done
clear
B)
fructose phosphatase
done
clear
C)
ribulose Diphosphate carboxylase
done
clear
D)
phosphoenol pyruvic acid carboxylase
done
clear
View Answer play_arrow
-
question_answer147) Mycorrhiza is an example of:
A)
endoparasitism
done
clear
B)
decomposers
done
clear
C)
symbiotic relationship
done
clear
D)
ectoparasitism
done
clear
View Answer play_arrow
-
question_answer148) Which one of the following is categorized under living fossils?
A)
Selaginella
done
clear
B)
Metasequoia
done
clear
C)
Pinus
done
clear
D)
Cycas
done
clear
View Answer play_arrow
-
question_answer149) Boron in green plants assists in:
A)
photosynthesis
done
clear
B)
sugar transport
done
clear
C)
activation of enzymes
done
clear
D)
acting as enzyme cofactor
done
clear
View Answer play_arrow
-
question_answer150) Chlorenchyma is known to develop in the:
A)
spore capsule of a moss
done
clear
B)
pollen tube of Pinus
done
clear
C)
cytoplasm of Chlarella
done
clear
D)
mycelium of a green mould such as Aspergillus
done
clear
View Answer play_arrow
-
question_answer151) The term "antibiotic" was coined by:
A)
Selman Waksman
done
clear
B)
Alexander Flemming
done
clear
C)
Edward Jenner
done
clear
D)
Louis Pasteur
done
clear
View Answer play_arrow
-
question_answer152) The aleurone layer in maize grain is specially rich in:
A)
lipids
done
clear
B)
auxins
done
clear
C)
proteins
done
clear
D)
starch
done
clear
View Answer play_arrow
-
question_answer153) Which one of the following sequences was proposed by Darwin and Wallace for organic evolution?
A)
Overproduction, constancy of population size, variations, natural selection
done
clear
B)
Variations, natural selection, overproduction, constancy of population size
done
clear
C)
Overproduction, variations, constancy of population size, natural selection
done
clear
D)
Variations, constancy of population size, overproduction, natural selection
done
clear
View Answer play_arrow
-
question_answer154) Given below are four matchings of an animal and its kind of respiratory organ: (A) silver fish?trachea (B) scorpion ? book lung (C) sea squirt ? pharyngeal gills (D) dolphin ? skin The correct matchings are:
A)
B and D
done
clear
B)
C and D
done
clear
C)
A and D
done
clear
D)
A, B and C
done
clear
View Answer play_arrow
-
question_answer155) Grey spots of oat are caused by deficiency of:
A)
Mn
done
clear
B)
Fe
done
clear
C)
Cu
done
clear
D)
Zn
done
clear
View Answer play_arrow
-
question_answer156) Which of the following discoveries resulted in a Nobel Prize?
A)
Recombination of linked genes
done
clear
B)
Genetic engineering
done
clear
C)
X-rays induce sex-linked recessive lethal mutations
done
clear
D)
Cytoplasmic inheritance
done
clear
View Answer play_arrow
-
question_answer157) The chief advantage of encystment of an Amoeba is:
A)
protection from parasites and predators
done
clear
B)
the chance to get rid of accumulated waste products
done
clear
C)
the ability to survive during adverse physical conditions
done
clear
D)
the ability to live for some time without ingesting food
done
clear
View Answer play_arrow
-
question_answer158) Which of the following plants are used as green manure in crop fields and in sandy soils?
A)
Saccharum munja and Lantana camara
done
clear
B)
Dichanthium annulatum and Azolla nilotica
done
clear
C)
Crotolaria juncea and Alhagi comelorum
done
clear
D)
Calotropis procera and Phyllanthus niruri
done
clear
View Answer play_arrow
-
question_answer159) In which kingdom would you classify the archaea and nitrogen-fixing organisms. If the five- kingdom system of classification is used:
A)
Protista
done
clear
B)
Monera
done
clear
C)
Plantae
done
clear
D)
Fungi
done
clear
View Answer play_arrow
-
question_answer160) Which one of the following traits of garden pea studied by Mendel was a recessive feature?
A)
Green pod colour
done
clear
B)
Round seed shape
done
clear
C)
Axial flower position
done
clear
D)
Green seed colour
done
clear
View Answer play_arrow
-
question_answer161) The genes controlling the seven pea characters studied by Mendel are now known to be located on how many different chromosome.
A)
Five
done
clear
B)
Four
done
clear
C)
Seven
done
clear
D)
Six
done
clear
View Answer play_arrow
-
question_answer162) Juicy hair-like structures observed in the lemon fruit develop from:
A)
endocarp
done
clear
B)
mesocarp and endocarp
done
clear
C)
exocarp
done
clear
D)
mesocarp
done
clear
View Answer play_arrow
-
question_answer163) Which one of the following describes correctly the homologous structures?
A)
Organs that have no function now, but had an important function in ancestors
done
clear
B)
Organs appearing only in embryonic stage and disappearing later in the adult
done
clear
C)
Organs with anatomical similarities, but performing different functions
done
clear
D)
Organs with anatomical dissimilarities, but performing same functions
done
clear
View Answer play_arrow
-
question_answer164) Darwin in his 'Natural Selection Theory', did not believe in any role of which one of the following in organic evolution?
A)
Struggle for existence
done
clear
B)
Discontinuous variations
done
clear
C)
Parasites and predators as natural enemies
done
clear
D)
Survival of the fittest
done
clear
View Answer play_arrow
-
question_answer165) During its life-cycle, Fasciola hepatica (liver fluke) infects its intermediate host and primary host at the following larval stages respectively:
A)
metacercaria and cercaria
done
clear
B)
miracidium and metacercaria
done
clear
C)
redia and miracidium
done
clear
D)
cercaria and redia
done
clear
View Answer play_arrow
-
question_answer166) Escherichia coli is used as an indicator organism to determine pollution of water with:
A)
industrial effluents
done
clear
B)
pollen of aquatic plants
done
clear
C)
heavy metals
done
clear
D)
faecal matter
done
clear
View Answer play_arrow
-
question_answer167) Down's syndrome is caused by an extra copy of chromosome number 21. What percentage of offspring produced by an affected mother and a normal father would be affected by this disorder?
A)
50%
done
clear
B)
25%
done
clear
C)
100%
done
clear
D)
75%
done
clear
View Answer play_arrow
-
question_answer168) What would happen if in a gene encoding a polypeptide of 50 amino acids, 25th codon (UAU) is mutated to UAA?
A)
A polypeptide of 49 amino acids will be formed
done
clear
B)
A polypeptide of 25 amino acids will be formed
done
clear
C)
A polypeptide of 24 amino acids will be formed
done
clear
D)
Two polypeptides of 24 and 25 amino acids will be formed
done
clear
View Answer play_arrow
-
question_answer169) Which one of the following pairs is not correctly matched?
A)
Vitamin \[{{B}_{12}}\] ? Pernicious anaemia
done
clear
B)
Vitamin \[{{B}_{1}}\] ? Beri-beri
done
clear
C)
Vitamin C ? Scurvy
done
clear
D)
Vitamin \[{{B}_{2}}\] ? Pellagra
done
clear
View Answer play_arrow
-
question_answer170) Which elements is located at the centre of the porphyrin ring in chlorophyll?
A)
Potassium
done
clear
B)
Manganese
done
clear
C)
Calcium
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
-
question_answer171) The major role of minor elements inside living organisms is to act as:
A)
constituents of hormones
done
clear
B)
binder of cell structure
done
clear
C)
co-factors of enzymes
done
clear
D)
building blocks of important amino acids
done
clear
View Answer play_arrow
-
question_answer172) In the genetic code dictionary, how many codons are used to code for all the 20 essential amino acids?
A)
61
done
clear
B)
60
done
clear
C)
20
done
clear
D)
64
done
clear
View Answer play_arrow
-
question_answer173) Which one of the following pairs of plants are not seed producers?
A)
Fiats and Chlamydomonas
done
clear
B)
Punica and Pinus
done
clear
C)
Fem and Funaria
done
clear
D)
Funaria and Ficus
done
clear
View Answer play_arrow
-
question_answer174) Systemic heart refers to:
A)
entire heart in lower vertebrates
done
clear
B)
the two ventricles together in humans
done
clear
C)
the heart that contracts under stimulation from nervous system
done
clear
D)
left auricle and left ventricle in higher vertebrates
done
clear
View Answer play_arrow
-
question_answer175) The linkage map of X-chromosome of fruit-fly has 66 units, with yellow body gene (y) at one end and bobbed hair (b) gene at the other end. The recombination frequency between these two genes (y and b) should be:
A)
\[\le \,50%\]
done
clear
B)
100%
done
clear
C)
66%
done
clear
D)
> 50%
done
clear
View Answer play_arrow
-
question_answer176) Choromosomes in a bacterial cell can be 1-3 in number and:
A)
can be either circular or linear, but never both within the same cell
done
clear
B)
can be circular as well as linear within die same cell
done
clear
C)
are always circular
done
clear
D)
are always linear
done
clear
View Answer play_arrow
-
question_answer177) Which one of the following contains the largest quantity of extracellular material?
A)
Stratified epithelium
done
clear
B)
Myelinated nerve fibres
done
clear
C)
Striated muscle
done
clear
D)
Areolar tissue
done
clear
View Answer play_arrow
-
question_answer178) Bundle of His is a network of:
A)
nerve fibres distributed in ventricles
done
clear
B)
nerve fibres found throughout the heart
done
clear
C)
muscle fibres distributed throughout the heart walls
done
clear
D)
muscle fibres found only in the ventricle wall
done
clear
View Answer play_arrow
-
question_answer179) Which endangered animal is the source of the world's finest, lightest, warmest and most expensive wool the shahtoosh?
A)
Kashmiri goat
done
clear
B)
Chiru
done
clear
C)
Nilgai
done
clear
D)
Cheetal
done
clear
View Answer play_arrow
-
question_answer180) Carcinoma refers to:
A)
malignant tumours of the colon
done
clear
B)
benign tumours of the connective tissue
done
clear
C)
malignant tumours of the connective tissue
done
clear
D)
malignant tumours of the skin or mucous membrane
done
clear
View Answer play_arrow
-
question_answer181) Which one of the following pairs correctly mathces a hormone with a disease resulting from its deficiency?
A)
Parathyroid hormone ? Tetani
done
clear
B)
Insulin ? Diabetes insipidus
done
clear
C)
Relaxin ? Gigantism
done
clear
D)
Prolactin ? Cretinism
done
clear
View Answer play_arrow
-
question_answer182) Maximum application of animals cell culture technology today is in the production of:
A)
vaccines
done
clear
B)
edible proteins
done
clear
C)
insulin
done
clear
D)
interferons
done
clear
View Answer play_arrow
-
question_answer183) Ommatidia serve die purpose of photoreception in:
A)
humans
done
clear
B)
sunflower
done
clear
C)
cockroach
done
clear
D)
frog
done
clear
View Answer play_arrow
-
question_answer184) In a random mating population in equilibrium, which of die following brings about a change in gene frequency in non-directional manner?
A)
Selection
done
clear
B)
Migration
done
clear
C)
Mutations
done
clear
D)
Random drift
done
clear
View Answer play_arrow
-
question_answer185) In which one of the following options the two names refer to one and the same thing?
A)
Citric acid cycle and Calvin cycle
done
clear
B)
Tricarboxylic acid cycle and urea cycle
done
clear
C)
Krebs cycle and Calvin cycle
done
clear
D)
Tricarboxylic acid cycle and citric acid cycle
done
clear
View Answer play_arrow
-
question_answer186) Test tube baby means a baby born when:
A)
the ovum is fertilized externally ad thereafter implanted in the uterus
done
clear
B)
it develops from a non-fertilized egg
done
clear
C)
it is developed in a test tube
done
clear
D)
it is developed through tissue culture method
done
clear
View Answer play_arrow
-
question_answer187) Which one of the following bacteria has found extensive use in genetic engineering work plants?
A)
Bacillus coagulens
done
clear
B)
Agrobacterium tumefaciens
done
clear
C)
Costridium septicum
done
clear
D)
Xanthomonas citri
done
clear
View Answer play_arrow
-
question_answer188) Which group of vertebrates comprises the highest number of endangered species?
A)
Reptiles
done
clear
B)
Birds
done
clear
C)
Mammals
done
clear
D)
Fishes
done
clear
View Answer play_arrow
-
question_answer189) During transcription, the DNA site at which RNA polymerase binds is called:
A)
receptor
done
clear
B)
enhancer
done
clear
C)
promoter
done
clear
D)
regulator
done
clear
View Answer play_arrow
-
question_answer190) During embryonic development, the establishment of polarity along anterior posterior, dorsal/ventral or medial/lateral axis is called:
A)
anamorphosis
done
clear
B)
pattern formation
done
clear
C)
organizer phenomena
done
clear
D)
axis formation
done
clear
View Answer play_arrow
-
question_answer191) What does ?lac? refer to in what we call the lac operon?
A)
Lac insect
done
clear
B)
The number, 1, 00, 000
done
clear
C)
Lactose
done
clear
D)
Lactase
done
clear
View Answer play_arrow
-
question_answer192) Two crosses between the same pair of genotypes or phenotypes in which the sources of the gametes are reversed in one cross, is known as:
A)
dihybrid cross
done
clear
B)
reverse cross
done
clear
C)
test cross
done
clear
D)
reciprocal cross
done
clear
View Answer play_arrow
-
question_answer193) Pattern baldness, moustaches and beard in human males are examples of:
A)
sex differentiating traits
done
clear
B)
sex determining traits
done
clear
C)
sex linked traits
done
clear
D)
sex limited traits
done
clear
View Answer play_arrow
-
question_answer194) Which one of the following conditions though harmful in itself, is also a potential saviour from a mosquito home infectious disease?
A)
Pernicious anaemia
done
clear
B)
Leukemia
done
clear
C)
Thalassaemia
done
clear
D)
Sickle cell anaemia
done
clear
View Answer play_arrow
-
question_answer195) Plants reproducing by spores such as mosses and ferns are grouped under the general term:
A)
sporophytes
done
clear
B)
thallophytes
done
clear
C)
cryptogams
done
clear
D)
bryophytes
done
clear
View Answer play_arrow
-
question_answer196) Which one pair of examples will correctly represent the grouping spermatophyta according to one of the schemes of classifying plants?
A)
Rhizopus, Triticum
done
clear
B)
Ginkgo, Pisum
done
clear
C)
Acacia, sugarcane
done
clear
D)
Pinus, Cycas
done
clear
View Answer play_arrow
-
question_answer197) Nicotiana sylvestris flowers only during long days and N. tabacum flowers only during short days. If raised in the laboratory under different photoperiods, they can be induced to flower at the same time and can be crossfertilized to produce s elf-fertile offspring. What is die best reason for considering N. sylvestris and N. tabacum to be separate species?
A)
They are physiologically distinct
done
clear
B)
They are morphologically distinct
done
clear
C)
They cannot interbreed in nature
done
clear
D)
They are reproductively distinct
done
clear
View Answer play_arrow
-
question_answer198) Biosystematics aims at:
A)
the classification of organisms based on their evolutionary history and establishing their phylogeny on the totality of various parameters from all fields of studies
done
clear
B)
identification and arrangement of organisms on the basis of their cytological characterisitcs
done
clear
C)
the classification of organisms based on broad morphological characters
done
clear
D)
delimiting various taxa of organisms and establishing their relationships
done
clear
View Answer play_arrow
-
question_answer199) Bartholin's glands are situated:
A)
on either side of vagina in humans
done
clear
B)
on either side of vas deference in humans
done
clear
C)
on the sides of the head of some amphibians
done
clear
D)
at the reduced tail end of birds
done
clear
View Answer play_arrow
-
question_answer200) Short-lived immunity acquired from mother to foetus across placenta or through mother's milk to the infant is categorised as:
A)
cellular immunity
done
clear
B)
innate non-specific immunity
done
clear
C)
active immunity
done
clear
D)
passive immunity
done
clear
View Answer play_arrow

 Choose the correct statement from the following:
Choose the correct statement from the following: