A) Z < 1 and attractive forces are dominant
B) Z < 1 and repulsive forces are dominant
C) Z > 1 and attractive forces are dominant
D) Z > 1 and repulsive forces are dominant
Correct Answer: A
Solution :
\[Z=\frac{pv}{\eta Rt}Z=\frac{{{V}_{real}}}{rideal}\]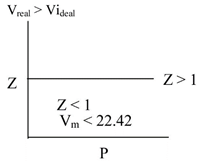 Z < 1 then intenvolealav forcen dominant Z > 1 then repulsive forces dominate
Z < 1 then intenvolealav forcen dominant Z > 1 then repulsive forces dominate
You need to login to perform this action.
You will be redirected in
3 sec
