A) Aryl amines are generally less basic than alkyl amines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring electron system.
B) Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring electron system.
C) Arylamines are generally more basic than alkylamines because of aryl group.
D) Arylamines are generally more basic than alkylamines, because the nitrongen atom in arylamines is sp-hybridized.
Correct Answer: A
Solution :
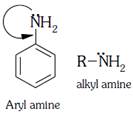 Delocalized lone pair of nitrogen less basic
Delocalized lone pair of nitrogen less basic
You need to login to perform this action.
You will be redirected in
3 sec
