| A. \[~C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\text{ }+\text{ }KOH\to \] \[C{{H}_{3}}CH=C{{H}_{2}}+KBr\text{ }+\text{ }{{H}_{2}}O\] |
| B. |
C. 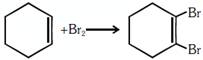 |
A) (A) and (b) are elimination reaction and (C) is addition reaction
B) (A) is elimination, (B) is substitution and (C) is addition reaction
C) (A) is elimination, (B) and (C) are substitution reactions
D) (A) is substitution, (B) and (C) are addition reaction
Correct Answer: B
Solution :
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br+KOH\to C{{H}_{3}}CH\] \[=C{{H}_{2}}+KBr+\text{ }{{H}_{2}}O\] breaking of \[2\sigma \] bonds and formation of 1 \[1\pi \] bond so it is an example of elimination reaction.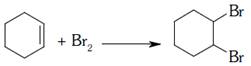 breaking of \[1\pi \] bond and formation of \[2\sigma \] bonds is addition reaction
breaking of \[1\pi \] bond and formation of \[2\sigma \] bonds is addition reaction
You need to login to perform this action.
You will be redirected in
3 sec
