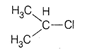
A)
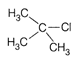
B)
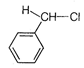
C)
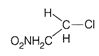
D)
Correct Answer: B
Solution :
The stability of carbocation follow the order \[{{3}^{\text{o}}}>{{2}^{\text{o}}}>{{1}^{\text{o}}}>\] methyl. More the number of alkyl group attached with the carbon atom carrying the positive charge greater would be the tendency to stabilize positive charge via inductive effect and hence more stable. (a)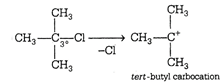 This structure is more stable due to nine \[\alpha \]-hydrogen and (nine hyperconjugative structures) three \[+I\] groups. (b)
This structure is more stable due to nine \[\alpha \]-hydrogen and (nine hyperconjugative structures) three \[+I\] groups. (b) 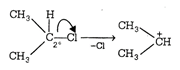 \[2{}^\circ \] carbocation containing 6 \[\alpha \]-hydrogen showing six hyperconjugative structure along with two \[+I\]group. (c)
\[2{}^\circ \] carbocation containing 6 \[\alpha \]-hydrogen showing six hyperconjugative structure along with two \[+I\]group. (c) 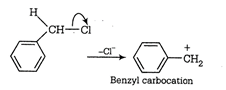 It has slightly lesser stability with \[{{3}^{\text{o}}}\]-alkyl carbocation due to presence of three electron donating alkyl group in \[{{3}^{\text{o}}}\]-alkyl carbocation. Although the stabilities of \[3{}^\circ \] and benzyl carbonium ion are almost same and cannot be compared in solution but whenever a comparison is made between Resonance (the cause of stability in benzyl carbonium ion) and No bond resonance (the cause of stability in \[{{3}^{\text{o}}}\]carbonium ion) then the former is always preferred hence here in this question benzyl carbonium ion is more stable than \[{{3}^{\text{o}}}\]carbonium ion. (d)
It has slightly lesser stability with \[{{3}^{\text{o}}}\]-alkyl carbocation due to presence of three electron donating alkyl group in \[{{3}^{\text{o}}}\]-alkyl carbocation. Although the stabilities of \[3{}^\circ \] and benzyl carbonium ion are almost same and cannot be compared in solution but whenever a comparison is made between Resonance (the cause of stability in benzyl carbonium ion) and No bond resonance (the cause of stability in \[{{3}^{\text{o}}}\]carbonium ion) then the former is always preferred hence here in this question benzyl carbonium ion is more stable than \[{{3}^{\text{o}}}\]carbonium ion. (d) 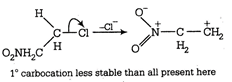
You need to login to perform this action.
You will be redirected in
3 sec
