A) \[{{[Co{{(CN)}_{6}}]}^{3-}}\]has no unpaired electrons and will be in a low-spin configuration.
B) \[{{[Co{{(CN)}_{6}}]}^{3-}}\] has four unpaired electrons and will be in a low-spin configuration.
C) \[{{[Co{{(CN)}_{6}}]}^{3-}}\] has four unpaired electrons and will be in a high-spin configuration.
D) \[{{[Co{{(CN)}_{6}}]}^{3-}}\] has no unpaired electrons and will be in a high-spin configuration.
Correct Answer: A
Solution :
\[{{[Co{{(CN)}_{6}}]}^{3-}}\] \[C{{o}^{3+}}=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{6}}\] \[C{{N}^{-}}\] is a strong field ligand and as it approaches the metal ion, the electrons must pair up. The splitting of the d-orbitals into two sets of orbitals in an octahedral \[{{[Co{{(CN)}_{6}}]}^{3-}}\] may be represented as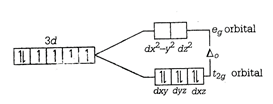 Here, for \[{{d}^{6}}\] ions, three elections first enter orbitals with parallel spin put the remaining may pair up in \[{{t}_{2g}}\] orbital giving rise to low spin complex (strong ligand) field. \[\therefore \,{{[Co{{(CN)}_{6}}]}^{3-}}\] has no unpaired electron and will be in a low spin configuration.
Here, for \[{{d}^{6}}\] ions, three elections first enter orbitals with parallel spin put the remaining may pair up in \[{{t}_{2g}}\] orbital giving rise to low spin complex (strong ligand) field. \[\therefore \,{{[Co{{(CN)}_{6}}]}^{3-}}\] has no unpaired electron and will be in a low spin configuration.
You need to login to perform this action.
You will be redirected in
3 sec
