A) 2, 4, 6-trinitrophenol
B) Benzoic acid
C) o-nitrophenol
D) Benzenesulphonic acid
Correct Answer: C
Solution :
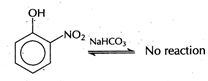 While 2, 4, 6-trinitrophenol, benzoic acid and benzene sulphonic acid are soluble in \[NaHC{{O}_{3}}\]. This reaction is possible in forward direction if acid is more acidic than\[{{H}_{2}}C{{O}_{3}}\]. O-nitrophenol is less acidic than\[{{H}_{2}}C{{O}_{3}}\]. Hence, it is not soluble in sodium hydrogen carbonate.
While 2, 4, 6-trinitrophenol, benzoic acid and benzene sulphonic acid are soluble in \[NaHC{{O}_{3}}\]. This reaction is possible in forward direction if acid is more acidic than\[{{H}_{2}}C{{O}_{3}}\]. O-nitrophenol is less acidic than\[{{H}_{2}}C{{O}_{3}}\]. Hence, it is not soluble in sodium hydrogen carbonate.
You need to login to perform this action.
You will be redirected in
3 sec
