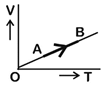
| The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is [NEET - 2018] |
 |
A) \[\frac{1}{3}\]
B) \[\frac{2}{3}\]
C) \[\frac{2}{5}\]
D) \[\frac{2}{7}\]
Correct Answer: C
Solution :
| [c] Given process is isobaric |
| \[\text{dQ=n}{{\text{C}}_{\text{p}}}\text{dT}\] |
| \[\text{dQ=n}\left( \frac{\text{5}}{\text{2}}\text{R} \right)\text{dT}\] |
| \[\text{dW=PdV=nRdT}\] |
| Required ratio \[\text{=}\frac{\text{dW}}{\text{dQ}}\text{=}\frac{\text{nRdT}}{\text{n}\left( \frac{\text{5}}{\text{2}}\text{R} \right)\text{dT}}\text{=}\frac{\text{2}}{\text{5}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
