question_answer 1) The observed wavelength of light coming from a distant galaxy is found to be increased by 0.5% as compared with that coming from a terrestrial source. The galaxy is
A)
stationary with respect to the earth
done
clear
B)
approaching the earth with velocity of light
done
clear
C)
receding from the earth with velocity of Light
done
clear
D)
receding from the earth with a velocity equal to \[1.5\times {{10}^{6}}m/s\]
done
clear
View Answer play_arrow
question_answer 2) A ball is moving in a circular path of radius 5 m. If tangential acceleration at any instant is 10 m/s2 and the net acceleration makes an angle 30 with the centripetal acceleration, then the instantaneous speed is
A)
\[50\sqrt{3}m/s\]
done
clear
B)
\[9.3m/s\]
done
clear
C)
\[6.6m/s\]
done
clear
D)
\[5.4m/s\]
done
clear
View Answer play_arrow
question_answer 3) A spherical solid ball of mass 1 kg and radius 3o cm is rotating about an axis passing through its centre with an angular velocity of 50 rad/s. The kinetic energy of rotation is
A)
4500 J
done
clear
B)
90 J
done
clear
C)
910 J
done
clear
D)
0.45J
done
clear
View Answer play_arrow
question_answer 4) The moment of inertia of a thin spherical shell of mass M and radius R about a diameter is \[\frac{2}{3}\]MR 2Its radius of gyration K about a tangent will be
A)
\[\sqrt{\frac{2}{3}}R\]
done
clear
B)
\[\frac{2}{3}R\]
done
clear
C)
\[\frac{5}{3}R\]
done
clear
D)
\[\sqrt{\frac{5}{3}}R\]
done
clear
View Answer play_arrow
question_answer 5) The outer sphere of a spherical air capacitor is earthed. For increasing its capacitance
A)
vacuum is created between two spheres
done
clear
B)
dielectric material is filled between the two spheres
done
clear
C)
the space between two spheres is increased
done
clear
D)
the earthing of the outer sphere is removed
done
clear
View Answer play_arrow
question_answer 6) A body weighs 200 N at the surface of earth. If it be placed in an artificial satellite revolving at height where acceleration due to gravity is half of that at earth's surface. It will weigh
A)
100 N
done
clear
B)
200 N
done
clear
C)
400 N
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 7) Bulk modulus of water is \[2\times {{10}^{9}}N/{{m}^{2}}.\] The change in pressure required to increase the density of water by 0.1% is
A)
\[2\times {{10}^{9}}N/{{m}^{2}}\]
done
clear
B)
\[2\times {{10}^{8}}N/{{m}^{2}}\]
done
clear
C)
\[2\times {{10}^{6}}N/{{m}^{2}}\]
done
clear
D)
\[2\times {{10}^{4}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 8) A simple pendulum is made by attaching a 1 kg bob to a 5 m long copper wire. Its period is 7 Now, if 1 kg bob is replaced by 10 kg bob, the period of oscillations
A)
remains T
done
clear
B)
becomes greater than T
done
clear
C)
becomes less than T
done
clear
D)
any of above depends on locality
done
clear
View Answer play_arrow
question_answer 9) A wire suspended vertically from one of its ends is stretched by attaching a weight of 200 N to the lower end. The weight stretches the wire by 1 mm. Then the elastic energy stored in the wire is
A)
20 J
done
clear
B)
0.1 J
done
clear
C)
0.2 J
done
clear
D)
10 J
done
clear
View Answer play_arrow
question_answer 10) A metal wire of length \[l\] area of cross-section A and Young's modulus Y behaves as a spring of spring constant k given by
A)
\[k=\frac{YA}{l}\]
done
clear
B)
\[k=\frac{2YA}{l}\]
done
clear
C)
\[k=\frac{YA}{2l}\]
done
clear
D)
\[k=\frac{Yl}{A}\]
done
clear
View Answer play_arrow
question_answer 11) The length of a seconds pendulum is
A)
99.8cm
done
clear
B)
99cm
done
clear
C)
100 cm
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 12) Certain neutron stars are believed to be rotating at about 1 rev /s. If such a star has a radius of 20 km, the acceleration of an object on the equator of the star will be
A)
\[20\times {{10}^{8}}m/{{s}^{2}}\]
done
clear
B)
\[8\times {{10}^{s}}m/{{s}^{2}}\]
done
clear
C)
\[120\times {{10}^{s}}m/{{s}^{2}}\]
done
clear
D)
\[4\times {{10}^{8}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 13) The length of a needle floating on water is 2.5 cm. Calculate the added force (due to surface tension) required to pull the needle out of water. (Surface tension of water \[=7.2\times {{10}^{-2}}N/{{m}^{2}}\])
A)
\[3.6\times {{10}^{-3}}N\]
done
clear
B)
\[3.4\times {{10}^{-10}}N\]
done
clear
C)
\[3.0\times {{10}^{-5}}N\]
done
clear
D)
\[3.8\times {{10}^{-11}}N\]
done
clear
View Answer play_arrow
question_answer 14) A capillary tube of radius r is immersed in water and water rises in it to a height H. Mass of water in the capillary tube is m. If the capillary of radius 2r is taken and dipped in water, the mass of water that will rise in the capillary tube will be
A)
m
done
clear
B)
2m
done
clear
C)
m/2
done
clear
D)
4m
done
clear
View Answer play_arrow
question_answer 15) In planetary motion the areal velocity of position vector of a planet depends on angular velocity (\[\omega \]) and distance of the planet from the sun (r). If so the correct relation for areal velocity is
A)
\[\frac{dA}{dt}\propto \,\omega r\]
done
clear
B)
\[\frac{da}{dt}\propto {{\omega }^{2}}r\]
done
clear
C)
\[\frac{da}{dt}\propto \omega {{r}^{2}}\]
done
clear
D)
\[\frac{da}{dt}\propto \sqrt{\omega r}\]
done
clear
View Answer play_arrow
question_answer 16) When a galvanometer is shunted by resistance S, its current capacity increases n times. If the same galvanometer is shunted by another resistance S', its current capacity will increase by n' is given by
A)
\[\frac{(n+1)S}{S'}\]
done
clear
B)
\[\frac{S(n-1)+S'}{S'}\]
done
clear
C)
\[\frac{n+S}{S'}\]
done
clear
D)
\[\frac{S(n-1)-S'}{S'}\]
done
clear
View Answer play_arrow
question_answer 17) A metallic solid sphere is rotating about its diameter as axes of rotation. If the temperature is increased by 200°C, the percentage increase in its moment of inertia is (Coefficient of linear expansion of the metal \[={{10}^{-5}}{{/}^{o}}C\])
A)
0.1%
done
clear
B)
0.2%
done
clear
C)
0.3%
done
clear
D)
0.4%
done
clear
View Answer play_arrow
question_answer 18) A 100 mH coil carries 1 A current. Energy stored in its magnetic field is
A)
0.1 J
done
clear
B)
0.05 J
done
clear
C)
0.05 J
done
clear
D)
1 J
done
clear
View Answer play_arrow
question_answer 19) Maximum energy is evolved during which of the following transitions?
A)
n = 1 to n =2
done
clear
B)
n =2 to n=6
done
clear
C)
n = 2to n =1
done
clear
D)
n= 6 to n=2
done
clear
View Answer play_arrow
question_answer 20) The average velocity of the molecules in a gas in equilibrium is
A)
proportional to\[\sqrt{T}\]
done
clear
B)
proportional to\[T\]
done
clear
C)
proportional to\[{{T}^{2}}\]
done
clear
D)
equal to zero
done
clear
View Answer play_arrow
question_answer 21) A vessel containing 0.1 m3 of air at 76 cm of Hg is connected to an evacuated vessel of capacity 0.09 m . The resultant air pressure is
A)
20 cm of Hg
done
clear
B)
30 cm of Hg
done
clear
C)
40 cm of Hg
done
clear
D)
60 cm of Hg
done
clear
View Answer play_arrow
question_answer 22) Two springs of force constant 1000 N/m and 2000 N/m are streched by same force. The ratio of their respective potential energies is
A)
2 : 1
done
clear
B)
1 : 2
done
clear
C)
4 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 23) If at the same temperature and pressure, the densities of two diatomic gases are \[{{d}_{1}}\] and \[{{d}_{2}}\] respectively, the ratio of mean kinetic energy per molecule of gases will be
A)
\[1:1\]
done
clear
B)
\[{{d}_{1}}:{{d}_{2}}\]
done
clear
C)
\[\sqrt{{{d}_{1}}}:\sqrt{{{d}_{2}}}\]
done
clear
D)
\[\sqrt{{{d}_{2}}}:\sqrt{{{d}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 24) When LED is forward biased, then
A)
electrons from the n-type material cross the -n junction and recombine with holes in the p- type material
done
clear
B)
electrons and holes neutralise each other
done
clear
C)
at junction electrons and holes remains at rest
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 25) High frequency waves are
A)
affected by the solar cycle
done
clear
B)
reflected by D2 layer
done
clear
C)
absorbed by the F2 layer
done
clear
D)
capable of use for long distance communication on the moon
done
clear
View Answer play_arrow
question_answer 26) Maximum velocity of photoelectron emitted is \[4.8m{{s}^{-1}}.\] If e/m ratio of electron is \[1.76\times {{10}^{11}}Ck{{g}^{-1}},\]then stopping potential is given by
A)
\[5\times {{10}^{-10}}J/C\]
done
clear
B)
\[3\times {{10}^{-7}}J/C\]
done
clear
C)
\[7\times {{10}^{-11}}J/C\]
done
clear
D)
\[2.5\times {{10}^{2}}J/C\]
done
clear
View Answer play_arrow
question_answer 27) A copper rod is suspended in a non homogeneous magnetic field region. The rod when in equilibrium will align itself
A)
in the region where magnetic field is strongest
done
clear
B)
in the region where magnetic field is weakest and parallel to direction of magnetic field there
done
clear
C)
in the direction in which it was originally suspended
done
clear
D)
in the region where magnetic field is weakest and perpendicular to the direction of magnetic field there
done
clear
View Answer play_arrow
question_answer 28) A black body of mass 34.38 g and surface area \[19.2c{{m}^{2}}\]is at an initial temperature of 400 K. It is allowed to cool inside an evacuated enclosure kept at constant temperature 300 K. The rate of cooling is 0.04°C/s. The specific heat of body is (Stefan's constant\[\sigma =5.73\times {{10}^{-8}}J{{m}^{-2}}{{K}^{-4}}\])
A)
2800 J/kg-K
done
clear
B)
2100 J Ag-K
done
clear
C)
1400 J/kg-K
done
clear
D)
1200 J/ kg-K
done
clear
View Answer play_arrow
question_answer 29) A pendulum suspended from the ceiling of a train has a period T when the train is at rest. When the train accelerates with a uniform acceleration a, the period of oscillation will
A)
increase
done
clear
B)
decrease
done
clear
C)
remain unaffected
done
clear
D)
become infinite
done
clear
View Answer play_arrow
question_answer 30) The motion of a particle executing SHM is given by \[x\] = 0.01 sin 100\[\pi \] (+0.05), where \[x\] is in metre and r in second. The time period of motion (in second) is
A)
0.01
done
clear
B)
0.02
done
clear
C)
0.1
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 31) What is the area of the plates of a 3F parallel plate capacitor, if the seperation between the plates is 5 mm?
A)
\[1.694\times {{10}^{9}}{{m}^{2}}\]
done
clear
B)
\[4.529\times {{10}^{9}}{{m}^{2}}\]
done
clear
C)
\[9.281\times {{10}^{9}}{{m}^{2}}\]
done
clear
D)
\[12.981\times {{10}^{9}}{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 32) When a certain volume of water is subjected to 100kPa pressure, the volume of water decreases by 0.005%. The speed of sound in water must be
A)
140 m/s
done
clear
B)
300 m/s
done
clear
C)
1400 m/s
done
clear
D)
5000 m/s
done
clear
View Answer play_arrow
question_answer 33) A man stands in a narrow, steep-sided valley. When he shouts he hears two echoes, one after 1 sand other after 2s. If the velocity of sound in air is 330 m/s, the width of the valley is
A)
330m
done
clear
B)
495m
done
clear
C)
660m
done
clear
D)
990m
done
clear
View Answer play_arrow
question_answer 34) A cylindrical tube, open at both ends emits a fundamental frequency\[f\]in air. The tube is dipped vertically in water, so that half of it is in water. The fundamental frequency of air column is now
A)
\[f/2\]
done
clear
B)
\[2f/4\]
done
clear
C)
\[f\]
done
clear
D)
\[2f\]
done
clear
View Answer play_arrow
question_answer 35) The end correction of a resonance column is cm. If the shortest length resonating with the tuning fork is 15.0 cm, the next resonating length will be
A)
31 cm
done
clear
B)
45 cm
done
clear
C)
46 cm
done
clear
D)
47 cm
done
clear
View Answer play_arrow
question_answer 36) A potentiometer has uniform potential gradient across it. Two cells connected in series to support each other and (ii) to oppose each other are balanced over 6m and 2m, respectively on the potentiometer wire. The emf s of the cells are in the ratio of
A)
1: 2
done
clear
B)
1 : 1
done
clear
C)
3 : 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 37) The de-Broglie wavelength of an electron having 80 eV of energy is nearly (\[1eV=1.6\times {{10}^{-19}}J,\]mass of electron \[=9.1\times {{10}^{-31}}kg,\] Planck's constant\[=6.6\times {{10}^{-34}}kg,s\])
A)
\[140{AA}\]
done
clear
B)
\[0.14{AA}\]
done
clear
C)
\[14{AA}\]
done
clear
D)
\[1.4{AA}\]
done
clear
View Answer play_arrow
question_answer 38) The distance between two coherent sources is mm. The screen is placed at a distance of 1 m from the sources. If the distance of the third bright fringe is 1.2 mm from the central fringe, the wavelength of light used is
A)
\[4000{AA}\]
done
clear
B)
\[5000{AA}\]
done
clear
C)
\[6000{AA}\]
done
clear
D)
\[7200{AA}\]
done
clear
View Answer play_arrow
question_answer 39) Two polaroids are crossed. If now one of them is rotated through 30° and unpolarised light of intensity \[{{l}_{o}}\] is incident on the first polaroid, then the intensity of transmitted light will be
A)
\[\frac{{{I}_{0}}}{4}\]
done
clear
B)
\[\frac{3{{I}_{0}}}{4}\]
done
clear
C)
\[\frac{3{{I}_{0}}}{8}\]
done
clear
D)
\[\frac{{{I}_{0}}}{8}\]
done
clear
View Answer play_arrow
question_answer 40) A wave front is represented by the plane\[y=3-x\]The propagation of wave takes place at
A)
\[45{}^\circ \] with x-direction
done
clear
B)
\[135{}^\circ \] with x-direction
done
clear
C)
\[60{}^\circ \] with x-direction
done
clear
D)
No sufficient data
done
clear
View Answer play_arrow
question_answer 41) The intensity ratio of two coherent sources of light is P. They are interfering in same region and produce interference pattern. Then the f, fringe visibility is
A)
\[\frac{1+p}{2\sqrt{p}}\]
done
clear
B)
\[\frac{1\sqrt{p}}{1+p}\]
done
clear
C)
\[\frac{p}{1+p}\]
done
clear
D)
\[\frac{2p}{1+p}\]
done
clear
View Answer play_arrow
question_answer 42) A condenser of capacitance 6 \[\mu F\] was originally charged to 10 V. Now potential difference is made 20 V. The increase in potential energy is
A)
\[3\times {{10}^{-4}}J\]
done
clear
B)
\[6\times {{10}^{-4}}J\]
done
clear
C)
\[9\times {{10}^{-4}}J\]
done
clear
D)
\[12\times {{10}^{-4}}J\]
done
clear
View Answer play_arrow
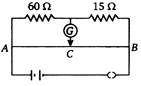
question_answer 43)
If there is no deflection in the galvanometer connected in a circuit shown in figure, then the ratio of lengths AC/CB is
A)
4 : 1
done
clear
B)
1 : 4
done
clear
C)
1:1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 44) In moving coil galvanometer, the magnetic field used is
A)
non-uniform
done
clear
B)
radial
done
clear
C)
uniform
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 45) A charged particle enters in a strong perpendicular magnetic field. Then its kinetic energy
A)
increases
done
clear
B)
decreases
done
clear
C)
remains constant
done
clear
D)
first increases and then becomes constant
done
clear
View Answer play_arrow
question_answer 46) Calculate the current which will produce a deflection of 30° in a tangent galvanometer, if its reduction factor is 3 A.
A)
1.732 A
done
clear
B)
0.732 A
done
clear
C)
3.732 A
done
clear
D)
2.732 A
done
clear
View Answer play_arrow
question_answer 47) Iron is ferromagnetic
A)
at all temperatures
done
clear
B)
at NTP only
done
clear
C)
above 770°C
done
clear
D)
below 770°C
done
clear
View Answer play_arrow
question_answer 48) The inductance of a coil in which a current of 0.2 A is increasing at the rate of 0.5 A/s represents a power flow of 0.5 W, is
A)
2H
done
clear
B)
5H
done
clear
C)
10 H
done
clear
D)
20 H
done
clear
View Answer play_arrow
question_answer 49) The mutual inductance of an induction coil is 5 H. In the primary coil, the current reduces from 5 A to zero in 10-3 s. What is the induced emf in the secondary coil?
A)
2500V
done
clear
B)
25000V
done
clear
C)
2510V
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 50) The electrical conductivity of semiconductor increases, when electromagnetic radiation of wavelength shorter than \[24800{AA}\] nm a incident on it. The band gap for the semiconductor is
A)
0.9 eV
done
clear
B)
0.7 eV
done
clear
C)
0.5 eV
done
clear
D)
1.1 eV
done
clear
View Answer play_arrow
question_answer 51) Wave front of a wave has direction with wave motion
A)
parallel
done
clear
B)
perpendicular
done
clear
C)
opposite
done
clear
D)
at an angle of \[\theta \]
done
clear
View Answer play_arrow
question_answer 52) A panicle rests on the top of a hemisphere of radius R. Find the smallest horizontal velocity that must be imparted to the panicle, if it is to leave the hemisphere without sliding down it a
A)
\[\sqrt{gR}\]
done
clear
B)
\[\sqrt{2gR}\]
done
clear
C)
\[\sqrt{3gR}\]
done
clear
D)
\[\sqrt{5gR}\]
done
clear
View Answer play_arrow
question_answer 53) Relative permeability of iron is 5500, then if magnetic susceptibility will be
A)
\[5500\times {{10}^{7}}\]
done
clear
B)
\[5500\times {{10}^{-7}}\]
done
clear
C)
5501
done
clear
D)
5499
done
clear
View Answer play_arrow
question_answer 54) The moment of inertia of a body about a given axis is 1.2 kg-m. Initially the body is at rest. If order to produce a rotational kinetic energy of 1500 J, an angular acceleration of 25 rad/s'2 must be applied about the axis for a duration of
A)
4s
done
clear
B)
2s
done
clear
C)
8s
done
clear
D)
10s
done
clear
View Answer play_arrow
question_answer 55) What is shape of magnet in moving coil galvanometer to make the radial magnetic field?
A)
Concave
done
clear
B)
Horse shoe magnet
done
clear
C)
Convex
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 56) The breaking strength of a rod of diameter 2cm is \[2\times {{10}^{5}}N.\] Then that for rod of same material but diameter 4 cm will be
A)
\[2\times {{10}^{5}}N\]
done
clear
B)
\[1\times {{10}^{5}}N\]
done
clear
C)
\[8\times {{10}^{5}}N\]
done
clear
D)
\[0.5\times {{10}^{5}}N\]
done
clear
View Answer play_arrow
question_answer 57) A metallic rod of Young's modulus \[2\times {{10}^{11}}N/{{m}^{2}}\]undergoes a strain of 0.5%. Then the energy stored per unit volume in the rod will be
A)
\[2.5\times {{10}^{-5}}J/{{m}^{3}}\]
done
clear
B)
\[5.0\times {{10}^{8}}J/{{m}^{3}}\]
done
clear
C)
\[2.5\times {{10}^{-8}}J/{{m}^{3}}\]
done
clear
D)
\[0.5\times {{10}^{11}}J/{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 58) A cyclotron can accelerate
A)
\[\beta \]-particles
done
clear
B)
\[\alpha \]-paiticles
done
clear
C)
high-velocity gamma rays
done
clear
D)
high velocity X-rays
done
clear
View Answer play_arrow
question_answer 59) The work done in increasing the size of a soap film from \[10cm\times 6cm\]to \[10cm\times 11cm\]is \[3\times {{10}^{-4}}J.\] The surface tension of the film is
A)
\[1.5\times {{10}^{-2}}N/m\]
done
clear
B)
\[3.0\times {{10}^{-2}}~N/m\]
done
clear
C)
\[~6.0{{\times }^{-2}}N/m\]
done
clear
D)
\[11\times {{10}^{-2}}~N/m\]
done
clear
View Answer play_arrow
question_answer 60) A potentiometer having the potential gradient of 2 mV/cm is used to measure the difference of potential across a resistance of\[10\Omega .\] If a length of 50 cm of the potentiometer wire is required to get the null points the current passing through 10\[\Omega \]resistor is (in mA)
A)
1
done
clear
B)
2
done
clear
C)
5
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 61) The concentration of\[A{{g}^{+}}\]ion in a given saturated solution of\[AgCl\]at\[25{}^\circ C\]is \[1.06\times {{10}^{-5}}g\,ion\text{ }{{L}^{-1}}\]. Thus, the solubility product of\[AgCl\]is
A)
\[0.453\times {{10}^{-10}}\]
done
clear
B)
\[0.530\times {{10}^{-10}}\]
done
clear
C)
\[1.12\times {{10}^{-10}}\]
done
clear
D)
\[2.12\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 62) Formic acid can be distinguished from acetic acid by its reaction with
A)
\[NaHC{{O}_{3}}\]
done
clear
B)
Tollen's reagent
done
clear
C)
\[NaOH\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 63) If a salt bridge is removed between the half cells, the voltage
A)
decreases to zero
done
clear
B)
increases
done
clear
C)
increases rapidly
done
clear
D)
do not change
done
clear
View Answer play_arrow
question_answer 64) Lanthanum the first element of lanthanide series has
A)
only unfilled 3d orbitals
done
clear
B)
unfilled 3d and 4d orbitals
done
clear
C)
unfilled 4d and\[4f\]orbitals
done
clear
D)
unfilled\[4f\]and Sd orbitals
done
clear
View Answer play_arrow
question_answer 65) Aspirin is prepared by the acetylation of salicylic acid with
A)
phenol
done
clear
B)
acetic anhydride
done
clear
C)
methyl acetate
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 66) A radioactive substance decays to,\[\frac{3}{4}\]th of its original value in 2h. The half-me of the substance is
A)
1 h
done
clear
B)
30 min
done
clear
C)
1 h 30 min
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 67) Among the following, the correct order of basicity is
A)
\[NH_{2}^{-}>O{{H}^{-}}>R{{O}^{-}}>RCO{{O}^{-}}\]
done
clear
B)
\[NH_{2}^{-}>R{{O}^{-}}>O{{H}^{-}}>RCO{{O}^{-}}\]
done
clear
C)
\[RCO{{O}^{-}}>NH_{2}^{-}>R{{O}^{-}}>O{{H}^{-}}\]
done
clear
D)
\[RCO{{O}^{-}}>R{{O}^{-}}>NH_{2}^{-}>O{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 68) The chemical reaction\[2{{O}_{3}}\xrightarrow{{}}3{{O}_{2}}\]proceeds as (i)\[{{O}_{3}}{{O}_{2}}+O\] (fast) (ii)\[O+{{O}_{3}}\xrightarrow{{}}2{{O}_{2}}\](slow) The rate expression should be
A)
\[r=k{{[{{O}_{3}}]}^{2}}\]
done
clear
B)
\[r=k{{[{{O}_{3}}]}^{2}}{{[{{O}_{2}}]}^{-1}}\]
done
clear
C)
\[r=k[{{O}_{3}}][{{O}_{2}}]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 69) Cannizaro reaction is not given by
A)
acetaldehyde
done
clear
B)
formaldehyde
done
clear
C)
benzaldehyde
done
clear
D)
triethyl acetaldehyde
done
clear
View Answer play_arrow
question_answer 70) Heat of combustion of\[C\]and\[CO\]are -394 and -285 kJ respectively, so heat of formation of \[CO\]in kJ/mol is
A)
-218
done
clear
B)
-109
done
clear
C)
+109
done
clear
D)
+218
done
clear
View Answer play_arrow
question_answer 71) Which enzyme converts glucose to ethanol?
A)
Diastase
done
clear
B)
Invertase
done
clear
C)
Zymase
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 72) The product formed is an isobar, if there is
A)
\[1\alpha \]emission
done
clear
B)
\[1\beta \]emission
done
clear
C)
\[1\alpha \]and\[1\beta \]emission
done
clear
D)
\[2\alpha \]and\[1\beta \]emission
done
clear
View Answer play_arrow
question_answer 73) Which of the following reaction follows\[{{S}_{N}}1\] mechanism?
A)
\[{{(C{{H}_{3}})}_{3}}C-C{{H}_{2}}Cl+C{{H}_{3}}OK\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHC{{H}_{2}}Cl+KCN\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}C-Cl+NaOH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHI+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following has more unpaired d-electrons?
A)
Zn
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[N{{i}^{3+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 75) How many structural isomers are possible for\[{{C}_{4}}{{H}_{9}}Cl\]?
A)
2
done
clear
B)
4
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 76) 0.5 molal solution of ethylene glycol in water is used as coolant in car. If the freezing point depression constant of water is\[1.86{}^\circ C\]per mol, the mixture will freeze at
A)
\[0.93{}^\circ C\]
done
clear
B)
\[-\text{ }0.93{}^\circ C\]
done
clear
C)
\[1.86{}^\circ C\]
done
clear
D)
\[-\text{ }1.86{}^\circ C\] \[\Rightarrow \]\[\Delta {{T}_{f}}={{k}_{f}}\times m\] \[=1.86\times 0.5=0.93{}^\circ C\] \[\therefore \] \[\Delta {{T}_{f}}=T_{f}^{o}-{{T}_{f}}\] \[\therefore \] \[{{T}_{f}}=T_{f}^{o}-\Delta {{T}_{f}}=0-0.93=-{{0.93}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 77) Solution of\[0.1N{{H}_{4}}OH\]and\[0.1N\,N{{H}_{4}}Cl\]has pH 9.25. Then,\[p{{K}_{b}}\]of\[N{{H}_{4}}OH\]is
A)
9.25
done
clear
B)
4.75
done
clear
C)
3.75
done
clear
D)
8.25
done
clear
View Answer play_arrow
question_answer 78)
A)
\[\text{ }\!\!\beta\!\!\text{ -}\]alanine
done
clear
B)
\[\alpha \text{-}\]alanine
done
clear
C)
ethylene diamine
done
clear
D)
\[\text{ }\!\!\gamma\!\!\text{ -}\]amino butyric acid
done
clear
View Answer play_arrow
question_answer 79) How many grams of\[C{{O}_{2}}\]will be produced by the complete combustion of 2 moles, of ethanol?
A)
132 g
done
clear
B)
44 g
done
clear
C)
176 g
done
clear
D)
88 g
done
clear
View Answer play_arrow
question_answer 80) In which of the following reactions ether does not form?
A)
\[{{C}_{2}}{{H}_{5}}ONa+{{C}_{2}}{{H}_{5}}I\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}ONa+{{(C{{H}_{3}})}_{3}}CBr\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}I+dry\,A{{g}_{2}}O\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}PH+{{H}_{2}}S{{O}_{4}}(140{}^\circ C)\]
done
clear
View Answer play_arrow
question_answer 81) Among the following sweetner which one has the lowest sweetness value?
A)
Alitame
done
clear
B)
Aspartame
done
clear
C)
Saccharine
done
clear
D)
Sucralose
done
clear
View Answer play_arrow
question_answer 82) In the Rosenmund's reaction \[RCOCl+{{H}_{2}}\xrightarrow{Pd/BaS{{O}_{4}}}RCHO+HCl\]here\[BaS{{O}_{4}}\]
A)
promotes catalytic activity of\[Pd\]
done
clear
B)
removes the\[HCl\]formed in the reaction
done
clear
C)
deactivates\[Pd\]
done
clear
D)
activates\[Pd\]
done
clear
View Answer play_arrow
question_answer 83) The first order rate constant for dissociation of\[{{N}_{2}}{{O}_{5}}\]is\[6.2\times {{10}^{-4}}{{s}^{-1}}\]. The half-life period (in s) of this dissociation will be
A)
1117.7
done
clear
B)
111.7
done
clear
C)
223.4
done
clear
D)
160.9
done
clear
View Answer play_arrow
question_answer 84)
The IUPAC name of the compound
A)
2-ethyl-2-methyl-3-hexanone
done
clear
B)
5-ethyl-5-methyl-4-hexanone
done
clear
C)
5,5-dimethyl-4-heptanone
done
clear
D)
3,3-dimethyl-4-heptanone
done
clear
View Answer play_arrow
question_answer 85) Amides are converted to amines by the following reaction
A)
Perkin
done
clear
B)
Claisen
done
clear
C)
Hofmann
done
clear
D)
Clemmensen
done
clear
View Answer play_arrow
question_answer 86) The gas phase reaction of nitric oxide and bromine yields nitrosyi bromide \[2NO(g)+B{{r}_{2}}(g)\xrightarrow{{}}2NOBr(g)\] The rate law is rate\[=k{{[NO]}^{2}}[B{{r}_{2}}]\] The overall reaction order is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 87) Fungicides are organic compounds of
A)
mercury
done
clear
B)
fluorine
done
clear
C)
lead
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 88) Reaction of ester with Grignard reagent give rise to
A)
primary alcohol
done
clear
B)
secondary alcohol
done
clear
C)
tertiary alcohol
done
clear
D)
ketone
done
clear
View Answer play_arrow
question_answer 89) The formula of nylon is
A)
\[-{{[OC{{(C{{H}_{2}})}_{6}}-CONH-{{(C{{H}_{2}})}_{4}}-NH-]}_{n}}\]
done
clear
B)
\[-{{[OC{{(C{{H}_{2}})}_{4}}-CONH-{{(C{{H}_{2}})}_{6}}-NH-]}_{n}}\]
done
clear
C)
\[-{{[OC{{(C{{H}_{2}})}_{3}}-CONH-{{(C{{H}_{2}})}_{6}}-NH-]}_{n}}\]
done
clear
D)
\[-{{[OC{{(C{{H}_{2}})}_{2}}-CONH-{{(C{{H}_{2}})}_{6}}-NH-]}_{n}}\]
done
clear
View Answer play_arrow
question_answer 90) \[CO\]binds itself to metal atoms through
A)
carbon atom only
done
clear
B)
oxygen atom only
done
clear
C)
carbon and oxygen atom
done
clear
D)
does not bind
done
clear
View Answer play_arrow
question_answer 91) Two molecules of acetone in presence of dil. \[NaOH\]give the product
A)
4-methyl-2-pentanone
done
clear
B)
4-hydroxy-2-pentanal
done
clear
C)
3-hydroxy isopropyl propanone
done
clear
D)
4-hydroxy-4-methyl-2-pentanone
done
clear
View Answer play_arrow
question_answer 92) In the periodic table actinides occupy
A)
III B group in 7th row
done
clear
B)
III B group in 6th row
done
clear
C)
IIIB group in 5th row
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 93) Diborane is used for reduction of
A)
carboxylic acids
done
clear
B)
esters
done
clear
C)
nitro groups
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 94) During electrochemical process
A)
Gibbs free energy increases
done
clear
B)
Gibbs free energy remains constant
done
clear
C)
no prediction can be made about Gibbs free energy
done
clear
D)
Gibbs free energy decreases
done
clear
View Answer play_arrow
question_answer 95) For endothermic reaction
A)
\[{{E}_{a}}>E_{a}^{'}\]
done
clear
B)
\[{{E}_{a}}=E_{a}^{'}\]
done
clear
C)
\[{{E}_{a}}<E_{a}^{'}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 96) At same temperature which pair of the following solutions are isotonic?
A)
\[0.2\text{ }M\text{ }BaC{{l}_{2}}\]and 0.2 M urea
done
clear
B)
0.1 M urea and\[0.1\text{ }M\text{ }NaCl\]
done
clear
C)
\[0.1\text{ }M\text{ }NaCl\]and\[0.1\text{ }M\,{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[0.1\text{ }M\text{ }Ba{{(N{{O}_{3}})}_{2}}\]and\[0.1\text{ }M\text{ }N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 97) \[R-OH+SOC{{l}_{2}}\xrightarrow{Pyridine}R-Cl+S{{O}_{2}}+HCl\]Pyridine in the above reaction
A)
catalyse the reaction
done
clear
B)
used to dissolve alkyl chloride
done
clear
C)
used to remove excess of\[SOC{{l}_{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 98) Which of the following is diamagnetic?
A)
\[CuC{{l}_{2}}\]
done
clear
B)
\[NiC{{l}_{2}}\]
done
clear
C)
\[FeC{{l}_{3}}\]
done
clear
D)
\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) Which of the following changes with change in temperature?
A)
Mole fraction
done
clear
B)
Formality
done
clear
C)
% (w/W)
done
clear
D)
Molality
done
clear
View Answer play_arrow
question_answer 100) Which of the following has least boiling point?
A)
Ethyl ether
done
clear
B)
n-propyl chloride
done
clear
C)
n-butyraldehyde
done
clear
D)
n-butyl alcohol
done
clear
View Answer play_arrow
question_answer 101) \[{{N}_{2}}\]gas is liberated when\[[HCl+NaN{{O}_{2}}]\]reacts with the following compound \[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\] Urea \[C{{H}_{3}}CON{{H}_{2}}\] \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\] The answer is
A)
a, b, c
done
clear
B)
a, b, d
done
clear
C)
a, c, d
done
clear
D)
b, c, d
done
clear
View Answer play_arrow
question_answer 102) The main constituent of most natural fibres is
A)
starch
done
clear
B)
glycol
done
clear
C)
cellulose
done
clear
D)
caprolactum
done
clear
View Answer play_arrow
question_answer 103) d(+) lactic acid is obtained from
A)
fermentation of cane sugar
done
clear
B)
green vegetables
done
clear
C)
muscles
done
clear
D)
fermentation of milk sugar
done
clear
View Answer play_arrow
question_answer 104) When\[_{13}^{27}Al\]is bombarded with a-particle, a radioactive isotope of phosphorus\[_{15}^{30}P\]is formed. Which particle is emitted along with\[_{15}^{30}P\]?
A)
Deuteron
done
clear
B)
Proton
done
clear
C)
Electron
done
clear
D)
Neutron
done
clear
View Answer play_arrow
question_answer 105) The difference between heat of reaction at constant pressure and constant volume of the reaction \[2{{C}_{6}}{{H}_{6}}(l)+15{{O}_{2}}(g)\xrightarrow{{}}12C{{O}_{2}}(g)\] \[+6{{H}_{2}}O(l)\]at\[25{}^\circ C\](in kJ) is
A)
\[-7.43\]
done
clear
B)
\[3.72\]
done
clear
C)
\[-3.72\]
done
clear
D)
\[7.43\]
done
clear
View Answer play_arrow
question_answer 106) Why\[Sc(Z=21)\]is not considered as transition element?
A)
Properties of\[Sc\]are similar to alkali metals
done
clear
B)
\[3d-\]orbitals are empty in its stable compound
done
clear
C)
Stable oxidation number of\[Sc\]is\[+2\]
done
clear
D)
Atomic volume of\[Sc\]is very large
done
clear
View Answer play_arrow
question_answer 107) Methyl isocyanide on hydrolysis gives
A)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
B)
\[HCOOH\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 108) With\[C{{H}_{3}}MgBr,\]diethyl ether gives
A)
coordination complex
done
clear
B)
n-butane
done
clear
C)
a mixture of ethyl bromide and methyl bromide
done
clear
D)
propane
done
clear
View Answer play_arrow
question_answer 109) Acetamide when heated with\[PC{{l}_{5}},\]gives
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[C{{H}_{3}}CN\]
done
clear
C)
\[C{{H}_{3}}CC{{1}_{2}}N{{H}_{2}}\]
done
clear
D)
\[CH{{C}_{12}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 110) The standard emf of a cell, involving one electron change is found to be 0.591 V at\[25{}^\circ C\]. The equilibrium constant of the reaction is (\[F=96500\text{ }C\text{ }mo{{l}^{-1}},\text{ }R=8.314\text{ }J{{K}^{-1}}mo{{l}^{-1}}\])
A)
\[1.0\times {{10}^{1}}\]
done
clear
B)
\[1.0\times {{10}^{5}}\]
done
clear
C)
\[1.0\times {{10}^{10}}\]
done
clear
D)
\[1.0\times {{10}^{30}}\]
done
clear
View Answer play_arrow
question_answer 111) For which reaction will\[\Delta H-\Delta E=0\]? Assume that each reaction is carried out in an open container.
A)
\[2CO(g)+{{O}_{2}}(g)\xrightarrow{{}}2C{{O}_{2}}(g)\]
done
clear
B)
\[PC{{l}_{5}}(g)\xrightarrow{{}}PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
C)
\[{{H}_{2}}(g)+B{{r}_{2}}(g)\xrightarrow{{}}2HBr(g)\]
done
clear
D)
\[C(s)+2{{H}_{2}}O(g)\xrightarrow{{}}2{{H}_{2}}(g)+C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 112) The type of bond that is most important in maintaining secondary structure of a protein is
A)
disulphide bridges
done
clear
B)
hydrogen bonding within the backbone
done
clear
C)
hydrogen bonding between R group
done
clear
D)
salt bridges
done
clear
View Answer play_arrow
question_answer 113) The angle\[\angle ROR\]is
A)
\[109{}^\circ 28'\]
done
clear
B)
\[95{}^\circ \]
done
clear
C)
\[110{}^\circ \]
done
clear
D)
\[105{}^\circ \]
done
clear
View Answer play_arrow
question_answer 114) The properties of\[Zr\]and\[Hf\]are same because
A)
they have similar radii
done
clear
B)
they belong to d-block
done
clear
C)
they have same valence electrons
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 115) Which of the following is added to food for the flavour of meat?
A)
Sodium benzoate
done
clear
B)
Sodium lactate
done
clear
C)
Sodium citrate
done
clear
D)
Sodium glutamate
done
clear
View Answer play_arrow
question_answer 116) The molarity of a\[0.2\text{ }N\text{ }N{{a}_{2}}C{{O}_{3}}\]solution will be
A)
0.05 M
done
clear
B)
0.2 M
done
clear
C)
0.4 M
done
clear
D)
0.1M
done
clear
View Answer play_arrow
question_answer 117) Which is not a colligative property?
A)
Refractive index
done
clear
B)
Lowering of vapour pressure
done
clear
C)
Depression in freezing point
done
clear
D)
Elevation in boiling point
done
clear
View Answer play_arrow
question_answer 118) \[RN{{O}_{2}}\]is reduced with\[Sn/HCl\]. Product formed is
A)
\[RNHOH\]
done
clear
B)
\[RN{{H}_{2}}\]
done
clear
C)
\[RNH_{3}^{+}\]
done
clear
D)
\[{{R}_{2}}NH\]
done
clear
View Answer play_arrow
question_answer 119) A bottle of cold drink contains 200 mL liquid in which\[C{{O}_{2}}\]is 0.1 molar. Suppose\[C{{O}_{2}}\]behaves like an ideal gas, the volume of the dissolved C02 at STP is
A)
0.224 L
done
clear
B)
22.4 L
done
clear
C)
0.448 L
done
clear
D)
44.8 L
done
clear
View Answer play_arrow
question_answer 120) If 8.0 g of a radioactive isotope has a half-life of 10 yr. The half-life of 2.0 g of the same substance is
A)
\[2.5\text{ }yr\]
done
clear
B)
\[5\,yr\]
done
clear
C)
\[20yr\]
done
clear
D)
\[10\text{ }yr\]
done
clear
View Answer play_arrow
question_answer 121) Proximal and distal convoluted tubules are parts of a
A)
nephron
done
clear
B)
oviduct
done
clear
C)
vas deferens
done
clear
D)
caecum
done
clear
View Answer play_arrow
question_answer 122) Heart sound dub is caused due to closing of
A)
valve
done
clear
B)
tricuspid valve
done
clear
C)
semilunar valve
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 123) Posterior pituitary gland
A)
produces and store hormones
done
clear
B)
stores 6 trophic hormones
done
clear
C)
stores and releases ADH and oxytocin
done
clear
D)
release and store growth and thyroid
done
clear
View Answer play_arrow
question_answer 124) stimulating hormone Inflammation response in allergy is caused by release of one of the following by mast cells
A)
antigenes
done
clear
B)
histamine
done
clear
C)
immunogenes
done
clear
D)
immunoglobulin
done
clear
View Answer play_arrow
question_answer 125) Phase of menstrual cycle when ovulation occurs in
A)
luteal
done
clear
B)
menstrual
done
clear
C)
proliferative
done
clear
D)
secretory
done
clear
View Answer play_arrow
question_answer 126) Process of maturation and development of sperms is called
A)
oogenesis
done
clear
B)
spermatogenesis
done
clear
C)
spermiogenesis
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 127) The instrument used for measuring blood pressure is
A)
electrocardiograph
done
clear
B)
X-rays
done
clear
C)
sphygmomanometer
done
clear
D)
electroencephalograph
done
clear
View Answer play_arrow
question_answer 128) Heart beat originates from
A)
pace-maker
done
clear
B)
cardiac muscles
done
clear
C)
left atrium
done
clear
D)
right ventricle
done
clear
View Answer play_arrow
question_answer 129) Cranial nerve showing maximum branching is
A)
trigeminal
done
clear
B)
vagus
done
clear
C)
optic
done
clear
D)
facial
done
clear
View Answer play_arrow
question_answer 130) AZT is the treatment of
A)
malaria
done
clear
B)
AIDS
done
clear
C)
TB
done
clear
D)
kala-azar
done
clear
View Answer play_arrow
question_answer 131) Gonadotrophins are secreted from
A)
gonads
done
clear
B)
anterior pituitary
done
clear
C)
posterior pituitary
done
clear
D)
thyroid
done
clear
View Answer play_arrow
question_answer 132) Earthworms are economically important for man as these are used as
A)
bait for catching fish
done
clear
B)
food of poultry
done
clear
C)
medicine for gout
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 133) Which of the following is a mismatch?
A)
Amphetamine?a stimulant
done
clear
B)
Morphine ? an opiate narcotic
done
clear
C)
LSD ? a hallucinogen
done
clear
D)
Cocaine ? a sedative/ tranquillizer
done
clear
View Answer play_arrow
question_answer 134) Which of the following is not related to sex chromosome X or Y?
A)
Turner's syndrome
done
clear
B)
Klinefelter's syndrome
done
clear
C)
Down's syndrome
done
clear
D)
Haemophilia and colour blindness
done
clear
View Answer play_arrow
question_answer 135) Which of the following is a pan of pectoral girdle?
A)
Ilium
done
clear
B)
Ischium
done
clear
C)
Acetabulum
done
clear
D)
Glenoid cavity
done
clear
View Answer play_arrow
question_answer 136) Water vascular system is characteristic of
A)
Protozoa
done
clear
B)
Porifera
done
clear
C)
Annelida
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 137) Taxonomically, which of the following set is matched correctly?
A)
Cattle fish, jelly fish, silver fish
done
clear
B)
Bat, pigeon, kite
done
clear
C)
Lobsters, spider, shrimps
done
clear
D)
Oyster, otter, Octopus
done
clear
View Answer play_arrow
question_answer 138) A normal woman is married with a man having hypertrichosis condition. They got one daughter and one son. What is the possibility of this daughter to have hypertrichosis condition?
A)
0%
done
clear
B)
25%
done
clear
C)
50%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 139) The centrum of eighth vertebra of frog is
A)
procoelous
done
clear
B)
heterocoelous
done
clear
C)
amphicoelous
done
clear
D)
opisrhocoelous
done
clear
View Answer play_arrow
question_answer 140) Which one of the following animals lay eggs yet the female secretes milk?
A)
Bat
done
clear
B)
Kangaroo
done
clear
C)
Platypus
done
clear
D)
Ostrich
done
clear
View Answer play_arrow
question_answer 141) In human eyes, colour perception is done by
A)
rod cells only
done
clear
B)
cone cells only
done
clear
C)
Both and
done
clear
D)
choroid layer cells
done
clear
View Answer play_arrow
question_answer 142) Which of the following embryonic membranes structure is excretory in function?
A)
Amnion
done
clear
B)
Allantois
done
clear
C)
Yolk sac
done
clear
D)
Vitelline chorion
done
clear
View Answer play_arrow
question_answer 143) Which of the following is a mismatch?
A)
Giraffe - Lamarck
done
clear
B)
Drosophila - Morgan
done
clear
C)
Galapagos island - Darwin's finches
done
clear
D)
Origin of species - Mendel
done
clear
View Answer play_arrow
question_answer 144) Which of the following type of cartilage is found in intervertebral disc of mammal?
A)
Hyaline cartilage
done
clear
B)
Fibrous cartilage
done
clear
C)
Calcified cartilage
done
clear
D)
Elastic cartilage
done
clear
View Answer play_arrow
question_answer 145) Archaeopteryx is
A)
a living fossil
done
clear
B)
a mammal
done
clear
C)
a connecting link between Annelida and Arthropoda
done
clear
D)
a connecting link between reptiles and birds
done
clear
View Answer play_arrow
question_answer 146) Which of the following set is a mis-match?
A)
Phosphoenol pyruvate carboxykinase ? Gluconeogenesis
done
clear
B)
Phospho fructokinase ? Glycolysis
done
clear
C)
Succinate dehydrogenase ? Kerbs cycle
done
clear
D)
Urease ? Urea cycle
done
clear
View Answer play_arrow
question_answer 147) A woman has a haemophilic son and three normal children. Her genotype and that of her husband with respect to this gene would be
A)
\[~XX\] and \[{{X}^{h}}Y\]
done
clear
B)
\[{{X}^{h}}{{X}^{h}}\]and \[{{X}^{h}}Y\]
done
clear
C)
\[{{X}^{h}}{{X}^{h}}\]and \[XY\]
done
clear
D)
\[{{X}^{h}}Y\]and \[XY\]
done
clear
View Answer play_arrow
question_answer 148) The causative agent of filaria is
A)
Wucherena bancrofti
done
clear
B)
Leishmania donovani
done
clear
C)
Plasmodium vivax
done
clear
D)
Trypanosoma gambiens
done
clear
View Answer play_arrow
question_answer 149) Pernicious anaemia is caused due to
A)
absence of vitamin-K
done
clear
B)
lackofvitamin-\[{{B}_{12}}\]
done
clear
C)
lack of vitamin-C
done
clear
D)
presence of intrinsic factor
done
clear
View Answer play_arrow
question_answer 150) How many molecules of oxygen can bind to a molecule of haemoglobin?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 151) Who proved that oxygen evolved in photosynthesis comes from water?
A)
Mayer
done
clear
B)
Calvin
done
clear
C)
Ruben, Hassid and Kamen
done
clear
D)
Blackman
done
clear
View Answer play_arrow
question_answer 152) \[2AD({{H}^{+}})\] produced during anaerobic glycolysis yield
A)
6 ATP molecules
done
clear
B)
4 ATP molecules
done
clear
C)
8 ATP molecules
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 153) Wilting of leaves at noon and their recovery toward evening is known as
A)
incipient wilting
done
clear
B)
temporary wilting
done
clear
C)
midday desiccation
done
clear
D)
permanent wilting
done
clear
View Answer play_arrow
question_answer 154) Which of the following induces and promotes cell division?
A)
ABA
done
clear
B)
Auxin
done
clear
C)
Cytokinin
done
clear
D)
Gibberellin
done
clear
View Answer play_arrow
question_answer 155) Artificial application of auxins like IAA, IBA and NAA to unpollinated pistils can form
A)
fruits with much flash
done
clear
B)
larger fruits
done
clear
C)
sweet fruits
done
clear
D)
seedless fruits
done
clear
View Answer play_arrow
question_answer 156) Vaccines prepared by genetic engineering are safe to man because they are
A)
least active form of virus
done
clear
B)
active form of virus
done
clear
C)
coat protein formed as antibody
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 157) Wildlife is destroyed most when
A)
there is lack of proper care
done
clear
B)
mass scale hunting for foreign trade
done
clear
C)
its natural habitat is destroyed
done
clear
D)
natural calamity
done
clear
View Answer play_arrow
question_answer 158) Intemodal elongation is stimulated by
A)
auxin
done
clear
B)
cytokinin
done
clear
C)
gibberellin
done
clear
D)
phenol
done
clear
View Answer play_arrow
question_answer 159) In the electron transport chain, in terminal oxidation, the cytochrome, which donates electrons to \[{{O}_{2}}\] is
A)
cytochrome-\[b\]
done
clear
B)
cytochrome-\[c\]
done
clear
C)
cytochrome-\[{{a}_{3}}\]
done
clear
D)
cytochrome-\[a\]
done
clear
View Answer play_arrow
question_answer 160) Stomata open or close due to the ion
A)
\[C{{a}^{2+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[~{{K}^{+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 161) Science of engineering and technology applied to life sciences is
A)
Biotechnology
done
clear
B)
Genetic engineering
done
clear
C)
Pathology
done
clear
D)
Biological science
done
clear
View Answer play_arrow
question_answer 162) Which of the following is generally used in chronic diarrhoea, dysentery, bleeding, piles, leucorrnoea etc?
A)
Quinine
done
clear
B)
Ephidrine
done
clear
C)
Chir
done
clear
D)
Cattha
done
clear
View Answer play_arrow
question_answer 163) Flash of light in dark inhibits flowering in
A)
SDP
done
clear
B)
LDP
done
clear
C)
LSDP
done
clear
D)
DNP
done
clear
View Answer play_arrow
question_answer 164) The growth of pollen tube towards embiyo sac is
A)
geotropism
done
clear
B)
chemotaxis
done
clear
C)
thigmotaxis
done
clear
D)
phototaxis
done
clear
View Answer play_arrow
question_answer 165) The specific character of \[{{C}_{4}}\]plants is
A)
bulli form cells
done
clear
B)
Kranz anatomy
done
clear
C)
parallel venation
done
clear
D)
isobilateral leaf
done
clear
View Answer play_arrow
question_answer 166) Triticum vulgare has been found to be presently evolved as
A)
diploid
done
clear
B)
tetraploid
done
clear
C)
pentaploid
done
clear
D)
hexaploid
done
clear
View Answer play_arrow
question_answer 167) Which of the following clogs the cavity of the xylem vessels?
A)
Tylosis
done
clear
B)
Cystolith
done
clear
C)
Hydathode
done
clear
D)
Raphide
done
clear
View Answer play_arrow
question_answer 168) Which of the following has multiflagellate sperms?
A)
Equisetum
done
clear
B)
Riccia
done
clear
C)
Lycopodium
done
clear
D)
Anthoceros
done
clear
View Answer play_arrow
question_answer 169) Which of the following is important source of edible protein?
A)
Spirogyra
done
clear
B)
Porphyra
done
clear
C)
spirulina
done
clear
D)
Cephaleuros
done
clear
View Answer play_arrow
question_answer 170) The character found only in halophytes is
A)
sunken stomata
done
clear
B)
vivipary
done
clear
C)
velamen tissue
done
clear
D)
heterophylly
done
clear
View Answer play_arrow
question_answer 171) A hyaline bisexual and self-fertilized flower that does not open at all is
A)
chasmogamaus
done
clear
B)
apogamous
done
clear
C)
cleistogamous
done
clear
D)
polygamous
done
clear
View Answer play_arrow
question_answer 172) Which one of the following is a pseudo carp?
A)
Apple
done
clear
B)
Guava
done
clear
C)
Tomato
done
clear
D)
Banana
done
clear
View Answer play_arrow
question_answer 173) Largest moss is
A)
Pogonatum
done
clear
B)
Funaria
done
clear
C)
Dawsonia
done
clear
D)
Polytrichum
done
clear
View Answer play_arrow
question_answer 174) Late blight of potato is caused by
A)
Cystopus
done
clear
B)
Phytophchora
done
clear
C)
Altenaria
done
clear
D)
Ustilago
done
clear
View Answer play_arrow
question_answer 175) The smallest free living organism is
A)
virus
done
clear
B)
mycoplasma
done
clear
C)
diatom
done
clear
D)
cyanobacterium
done
clear
View Answer play_arrow
question_answer 176) Name the fungus that is edible
A)
Penicillium
done
clear
B)
Mucor
done
clear
C)
Rhizopus
done
clear
D)
Morchella
done
clear
View Answer play_arrow
question_answer 177) The number of RNA molecules in 60S sub-particles of 80S ribosomes are
A)
five
done
clear
B)
four
done
clear
C)
three
done
clear
D)
two
done
clear
View Answer play_arrow
question_answer 178) The mutagenic agent among following is
A)
ethyl methane
done
clear
B)
ethylene
done
clear
C)
2,4-D
done
clear
D)
IAA
done
clear
View Answer play_arrow
question_answer 179) Ribosomal RNA (rRNA) is synthesized in
A)
nucleolus
done
clear
B)
nucleosome
done
clear
C)
cytoplasm
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 180) Plant length is increased by
A)
apical meristem
done
clear
B)
lateral meristem
done
clear
C)
dermatogen
done
clear
D)
periblem
done
clear
View Answer play_arrow
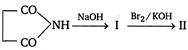 In the above sequence, II is
In the above sequence, II is
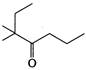 is
is
