| I. Increase in concentration of reactant increases the rate of a zero order reaction |
| II. Rate constant k is equal to collision frequency A, if \[{{E}_{a}}=0\] |
| III. Rate constant k is equal to collision frequency A, if \[{{E}_{a}}=\infty \] |
| IV. In k us T is a straight line |
| V. In k vs 1 /T is a straight line |
A) I and IV
B) II and V
C) III and IV
D) ll and III
Correct Answer: B
Solution :
The effect of temperature on rate constant is shown by Arrhenius equation. \[k=A{{e}^{-{{E}_{a}}/RT}}\] Here, if \[{{E}_{a}}=0\Rightarrow k=A\] The plot of In k vs\[\frac{1}{T}\] is a straight line and its slope will be\[-{{E}_{a}}/R\].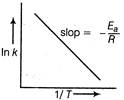 Hence, statements II and V are correct,
Hence, statements II and V are correct,
You need to login to perform this action.
You will be redirected in
3 sec
