question_answer 1) A beam of light composed of red arid green rays is incident obliquely at a point on the face of a rectangular glass slab. When coming out on the opposite parallel face, the red and green rays emerge from
A)
two points propagating in two different non-parallel directions
done
clear
B)
two points propagating in two different parallel directions
done
clear
C)
one point propagating in two different directions
done
clear
D)
one point propagating in the same direction
done
clear
View Answer play_arrow
question_answer 2) A photon moves to energy level\[{{E}_{1}}\]from\[{{E}_{2}}\]to find more stable nucleus, then the frequency will be
A)
exactly \[\frac{({{E}_{2}}-{{E}_{1}})}{h}\]
done
clear
B)
slightly greater than \[\frac{({{E}_{2}}-{{E}_{1}})}{h}\]
done
clear
C)
slightly less than\[\frac{({{E}_{2}}-{{E}_{1}})}{h}\]
done
clear
D)
\[hv\]
done
clear
View Answer play_arrow
question_answer 3) At\[273{}^\circ C,\]the emissive power of a perfect black body is R. What is its value at\[0{}^\circ C,\]?
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{R}{16}\]
done
clear
C)
\[\frac{R}{2}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 4) Among two discs A and B, first has radius 10 cm and charge\[{{10}^{-6}}C\]and second has radius 30 cm and charge\[{{10}^{-5}}C\]. When they are touched, charges on both are,\[{{q}_{A}}\]and\[{{q}_{B}}\] respectively, will be
A)
\[{{q}_{A}}=275\,\mu C,{{q}_{B}}=3.15\,\mu C\]
done
clear
B)
\[{{q}_{A}}=1.09\,\mu C,{{q}_{B}}=1.53\,\mu C\]
done
clear
C)
\[{{q}_{A}}={{q}_{B}}=5.5\,\mu C\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 5) The potential difference between the, cathode and the target electrode in a coolidge tube is 24.75 kV. The minimum wavelength of the emitted X-rays is
A)
\[0.1\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.5\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[5\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 6) A torque of\[{{10}^{-5}}Nm\]is required to hold a magnet at\[90{}^\circ \]with the horizontal component of the earths magnetic field. The torque required to hold it at\[30{}^\circ \]will be
A)
\[5\times {{10}^{-6}}Nm\]
done
clear
B)
\[\frac{1}{2}\times {{10}^{-5}}Nm\]
done
clear
C)
\[5\sqrt{3}\times {{10}^{-6}}Nm\]
done
clear
D)
Data is insufficient
done
clear
View Answer play_arrow
question_answer 7) \[A\text{ }220\text{ }V,100\text{ }W\]bulb is joined with a 110 V supply. The power consumed by the bulb is
A)
50 W
done
clear
B)
25 W
done
clear
C)
80 W
done
clear
D)
100 W
done
clear
View Answer play_arrow
question_answer 8) A sample of an ideal gas occupies a volume V at pressure p and absolute temperature T. The mass of each mdlecule is m, then the density of the gas is
A)
\[mkT\]
done
clear
B)
\[\frac{pm}{kT}\]
done
clear
C)
\[\frac{p}{km}\]
done
clear
D)
\[\frac{p}{kT}\]
done
clear
View Answer play_arrow
question_answer 9) An air column in a pipe which is closed at one end, will be in resonance with the vibrating body of frequency 166 Hz; if the length of the air column is
A)
0.5m
done
clear
B)
1.0m
done
clear
C)
1.5m
done
clear
D)
2.0m
done
clear
View Answer play_arrow
question_answer 10) A wave equation which gives the displacement along the direction is given by \[y=0,001\text{ }sin\text{ }(100r+x)\] where,\[x\] and y are in metre and\[t\]in second. This equation represents a wave
A)
travelling with a velocity of 100 m/s in the negative\[x-\]direction
done
clear
B)
travelling with a velocity of\[\frac{50}{\pi }\] m/s in the positive\[x-\]direction
done
clear
C)
of wavelength 1m
done
clear
D)
of frequency\[\frac{100}{\pi }\]Hz
done
clear
View Answer play_arrow
question_answer 11) The essential distinction between X-rays and \[\gamma -\]rays is that
A)
\[\gamma -\]rays have smaller wavelength than X-rays
done
clear
B)
\[\gamma -\]rays emanate from nucleus while X-rays emanate from outer part of the atom
done
clear
C)
\[\gamma -\]rays have greater ionizing power than X-rays
done
clear
D)
\[\gamma -\]rays are more penetrating than X-rays
done
clear
View Answer play_arrow
question_answer 12) Two particles, initially at rest move towards each other under the effect of gravitational force of attraction. At the instant when their relative velocity is 3v where, v is the velocity of the slower particle, then the speed of the centre of mass of two given particles is
A)
\[1v\]
done
clear
B)
\[2v\]
done
clear
C)
\[3v\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 13) A body of mass 10 kg moves with a velocity\[v\] of 2 m/s along a circular path of radius 8 m. The power produced by the body will be
A)
\[10J/s\]
done
clear
B)
\[98J/s\]
done
clear
C)
\[49J/s\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 14) A ball of mass 0.5 kg is moving with a velocity \[v\]of 2 m/s. It is subjected to a force of\[x\] newton in 2 s. Because of this force, the ball moves with velocity of 3 m/s. The value of\[x\] is
A)
5 N
done
clear
B)
8.25 N
done
clear
C)
0.25 N
done
clear
D)
1.0 N
done
clear
View Answer play_arrow
question_answer 15) A thin prism\[{{P}_{1}}\] with angle\[4{}^\circ \]and made from glass of refractive index 1.54 is combined with another prism\[{{P}_{2}}\]made of glass of refractive index 1.72 to produce dispersion without deviation. The angle of prism\[{{P}_{2}}\]is
A)
\[5.33{}^\circ \]
done
clear
B)
\[4{}^\circ \]
done
clear
C)
\[2.6{}^\circ \]
done
clear
D)
\[3{}^\circ \]
done
clear
View Answer play_arrow
question_answer 16) In a reaction\[_{92}B{{e}^{234}}{{\xrightarrow[{}]{{}}}_{88}}{{Y}^{218}},\]the number of\[\alpha \]and\[\beta -\]particles emitted respectively, are
A)
4, 4
done
clear
B)
4, 6
done
clear
C)
4, 8
done
clear
D)
4, 2
done
clear
View Answer play_arrow
question_answer 17) In the following transitions, which one has higher frequency?
A)
\[3\to 1\]
done
clear
B)
\[4\to 2\]
done
clear
C)
\[4\to 3\]
done
clear
D)
\[3\to 2\]
done
clear
View Answer play_arrow
question_answer 18) If increase in linear momentum of a body is 50%, then change in its kinetic energy is
A)
25%
done
clear
B)
125%
done
clear
C)
150%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 19) The current gain\[\alpha \]of a transistor in common-base mode is 0.995. Its current gain P in the common-emitter mode is
A)
200
done
clear
B)
90.5
done
clear
C)
100
done
clear
D)
1.005
done
clear
View Answer play_arrow
question_answer 20) A photocell is illuminated by a small bright Source placed 2 m away. When the same source of light is placed 4m away, the electrons emitted by photo-cathode in one second
A)
carry one quarter of their previous energy
done
clear
B)
carry one quarter of their previous momentum
done
clear
C)
are half numerous
done
clear
D)
are one quarter numerous
done
clear
View Answer play_arrow
question_answer 21) Two thin long parallel wires separated by a distance b are carrying a current i ampere each. The magnitude of the force per unit length exerted by one wire on the other, is
A)
\[\frac{{{\mu }_{0}}{{i}^{2}}}{{{b}^{2}}}\]
done
clear
B)
\[\frac{{{\mu }_{0}}i}{2\pi {{b}^{2}}}\]
done
clear
C)
\[\frac{{{\mu }_{0}}i}{2\pi b}\]
done
clear
D)
\[\frac{{{\mu }_{0}}{{i}^{2}}}{2\pi b}\]
done
clear
View Answer play_arrow
question_answer 22) A conducting wire of cross-sectional area 1 \[c{{m}^{2}}\]has\[3\times {{10}^{23}}\]charge carriers per\[metr{{e}^{3}}\]. If wire carries a current 24 mA, then rift velocity of carriers is
A)
\[5\times {{10}^{-2}}m/s\]
done
clear
B)
\[0.5\text{ }m/s\]
done
clear
C)
\[5\times {{10}^{-3}}\text{ }m/s\]
done
clear
D)
\[5\times {{10}^{-6}}\text{ }m/s\]
done
clear
View Answer play_arrow
question_answer 23) The capacitance of a metallic sphere is\[1\mu F,\]then its radius is nearly
A)
1.11 m
done
clear
B)
10 m
done
clear
C)
9 km
done
clear
D)
1.11 cm
done
clear
View Answer play_arrow
question_answer 24) For a projectile\[{{(range)}^{2}}\]is 48 times of \[{{(maximum\text{ }height)}^{2}}\]obtained. Find the angle of projection.
A)
\[60{}^\circ \]
done
clear
B)
\[30{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[75{}^\circ \]
done
clear
View Answer play_arrow
question_answer 25) At room temperature, the rms speed of the molecules of a certain diatomic gas is found to be 1933 m/s. The gas is
A)
\[{{H}_{2}}\]
done
clear
B)
\[{{F}_{2}}\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 26) The equation of longitudinal wave is represented as\[y=20\text{ }cos\pi (50t-x)cm\]. Then its wavelength is
A)
120 cm
done
clear
B)
50 cm
done
clear
C)
2 cm
done
clear
D)
5 cm
done
clear
View Answer play_arrow
question_answer 27) With the increase of temperature, the surface tension of the liquid
A)
may increase or decrease depending on. the density of the liquid
done
clear
B)
remains the same
done
clear
C)
always increases
done
clear
D)
always decreases
done
clear
View Answer play_arrow
question_answer 28) A force of\[6\times {{10}^{6}}N{{m}^{-2}}\] required for breaking a material. The density p of the material is\[3\times {{10}^{3}}kg\text{ }{{m}^{-3}}\]. If the wire is to break under its own weight, the length of the wire made of that material should be (take\[g=10\text{ }m{{s}^{-2}}\])
A)
20 m
done
clear
B)
200 m
done
clear
C)
100 m
done
clear
D)
2000 m
done
clear
View Answer play_arrow
question_answer 29) A ball falls from 20 m height on floor and rebounds to 5 m. Time of contact is 0.02 s. Find acceleration during impact.
A)
\[1200\text{ }m/{{s}^{2}}\]
done
clear
B)
\[1000\text{ }m/{{s}^{2}}\]
done
clear
C)
\[2000\text{ }m/{{s}^{2}}\]
done
clear
D)
\[1500\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 30) Two charges are at a distance d apart. If a copper plate of thickness\[\frac{d}{2}\]is kept between them, the effective force will be
A)
\[\frac{F}{2}\]
done
clear
B)
zero
done
clear
C)
\[2F\]
done
clear
D)
\[\sqrt{2F}\]
done
clear
View Answer play_arrow
question_answer 31) Two mirrors are placed at right angle to each other. A man is standing between them combing his hair. How many images will he see?
A)
2
done
clear
B)
3
done
clear
C)
1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 32) 15 g of ice melts to form water at\[0{}^\circ C\]. What is the change in entropy?
A)
18.5
done
clear
B)
15
done
clear
C)
zero
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) A small magnet kept in a non-uniform magnetic field experiences
A)
neither a force nor a torque
done
clear
B)
a force and a torque
done
clear
C)
a torque but not a force
done
clear
D)
a force but not a torque
done
clear
View Answer play_arrow
question_answer 34) A particle is executing SHM at mid point of mean position and extremity. What is the potential energy in terms of total energy
A)
\[\frac{E}{4}\]
done
clear
B)
\[\frac{E}{16}\]
done
clear
C)
\[\frac{E}{2}\]
done
clear
D)
\[\frac{E}{8}\]
done
clear
View Answer play_arrow
question_answer 35) In case of steel wire or a metal wire, the elastic limit is reached when
A)
the wire just break
done
clear
B)
the load is more than the weight of wire
done
clear
C)
elongation is inversely proportional to the tension
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 36) An eraser weighing\[1N\]is pressed against a vertical black board with a normal force of 5 N. The coefficient of friction\[\mu \]between eraser and Haclchoard is approximately 0.4. The force along the blackboard required to move the eraser is
A)
\[2N\]
done
clear
B)
\[0.4N\]
done
clear
C)
\[2.4N\]
done
clear
D)
\[9.8N\]
done
clear
View Answer play_arrow
question_answer 37) A ball of mass 0.12 kg is being whirled in a horizontal circle at the end of string 0.5 m long. It incapable of making 231 revolutions in one minute. The breaking tension of the string is
A)
3N
done
clear
B)
15.1 N
done
clear
C)
31.5 N
done
clear
D)
35.1 N
done
clear
View Answer play_arrow
question_answer 38) A wire of length I and resistance R is stretched to get the radius of cross-section\[\frac{r}{2}\]. Then the new value of R is
A)
16R
done
clear
B)
4R
done
clear
C)
8R
done
clear
D)
5R
done
clear
View Answer play_arrow
question_answer 39) Calcium plate has maximum possible radiation of wavelength\[\lambda \]of 400 nm to eject electrons. Its work function is
A)
2.3 eV
done
clear
B)
3.1 eV
done
clear
C)
4.5 eV
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40) If one face of prism is silvered having prism angle\[30{}^\circ \]and\[\mu =\sqrt{2}.\]What will be the angle of incidence, so that the incident ray retraces its path?
A)
\[30{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 41) A\[1\mu F\]capacitor is charged to 50 V potential difference and then discharged through a 10 mH inductor of negligible resistance. The maximum current in the inductor will be
A)
0.5 A
done
clear
B)
1.6 A
done
clear
C)
0.16 A
done
clear
D)
1.0 A
done
clear
View Answer play_arrow
question_answer 42) Dimensions of capacitance is.
A)
\[[{{M}^{-1}}{{L}^{-2}}{{T}^{4}}{{A}^{2}}]\]
done
clear
B)
\[[ML{{T}^{-3}}{{A}^{-1}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-3}}{{A}^{-1}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{-2}}{{T}^{3}}{{A}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 43) In a mechanical refrigerator, the low temperature coils are at a temperature of \[-23{}^\circ C\] and the compressed gas in the condenser has a temperature of\[27{}^\circ C\]. The theoretical coefficient of performance is
A)
5
done
clear
B)
8
done
clear
C)
6
done
clear
D)
10
done
clear
View Answer play_arrow
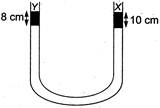
question_answer 44)
A liquid X of density\[3.36\text{ }g/c{{m}^{3}}\]is poured in a U-tube, which contains Hg. Another liquid Y is poured in left arm with height 8 cm, upper levels of X and Y are same. What is the density of Y?
A)
0.8 g/cc
done
clear
B)
1.2 g/cc
done
clear
C)
1.4 g/cc
done
clear
D)
1.6 g/cc
done
clear
View Answer play_arrow
question_answer 45) For a given material, the Youngs modulus is 2.4 times that of (rigidity modulus, then Poissons ratio is
A)
0.2
done
clear
B)
0.4
done
clear
C)
4.2
done
clear
D)
2.4
done
clear
View Answer play_arrow
question_answer 46) A ball thrown vertically upwards with an initial velocity\[1.4\text{ }m{{s}^{-1}}\]returns in 2 s. The total displacement of the ball is
A)
22.4cm
done
clear
B)
zero
done
clear
C)
44.8m
done
clear
D)
33.6m
done
clear
View Answer play_arrow
question_answer 47) In double slit experiment, the angular width of the fringes is\[0.20{}^\circ \] for the sodium light \[(\lambda =5890\overset{o}{\mathop{\text{A}}}\,)\]. In order to increase the angular width of the fringes by 10%, the necessary change in wavelength is
A)
zero
done
clear
B)
increased by \[6479\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
decreased by \[589\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
increased by \[589\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 48) A convex lens has mean focal length of 20 cm. The dispersive power of the material of the lens is 0.02. The longitudinal , chromatic aberration for an object at infinity is
A)
\[{{10}^{3}}\]
done
clear
B)
0.80
done
clear
C)
0.40
done
clear
D)
0.20
done
clear
View Answer play_arrow
question_answer 49) 1 mg gold undergoes decay with 2.7 days half-life period, amount left after 8.1 days is
A)
0.125 mg
done
clear
B)
0.5 mg
done
clear
C)
0.25 mg
done
clear
D)
0.91 mg
done
clear
View Answer play_arrow
question_answer 50) A planet has same density and same acceleration due to gravity as of earth and universal gravitational constant G is twice of earth. The ratio of their radii is
A)
\[1:4\]
done
clear
B)
\[1:5\]
done
clear
C)
\[1:2\]
done
clear
D)
\[3:2\]
done
clear
View Answer play_arrow
question_answer 51) If refractive index of glass is 1.50 and of water is 1.33, then critical angle is
A)
\[{{\sin }^{-1}}\left( \frac{8}{9} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{2}{3} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{8}{9} \right)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 52) The ionisation potential of mercury is 10.39 V. How far ah electron must travel in an electric field of\[1.5\times {{10}^{6}}V/m\]to gain sufficient energy to ionise mercury?
A)
\[\frac{10.39}{1.6\times {{10}^{-19}}}m\]
done
clear
B)
\[\frac{10.39}{2\times 1.6\times {{10}^{-19}}}m\]
done
clear
C)
\[10.39\times 1.6\times {{10}^{-19}}m\]
done
clear
D)
\[\frac{10.39}{1.5\times {{10}^{6}}}m\]
done
clear
View Answer play_arrow
question_answer 53) A straight wire conductor of length\[l\]of 0.4 m is moving with a speed\[v\]of 7 m/s perpendicular to a magnetic field B of intensity\[0.9\text{ }Wb/{{m}^{2}}\]. The induced emf across the conductor is
A)
2.52V
done
clear
B)
25.2V
done
clear
C)
5.26V
done
clear
D)
1.26V
done
clear
View Answer play_arrow
question_answer 54) A cylindrical tube closed at one end contains air. It produces the fundamental note of frequency 512 Hz. If the tube is opened at both ends, the fundamental frequency that can be excited is
A)
256 Hz
done
clear
B)
512 Hz
done
clear
C)
1024 Hz
done
clear
D)
128 Hz
done
clear
View Answer play_arrow
question_answer 55) A block of steel of size\[5\text{ }cm\times 5cm\times 5\text{ }cm\]is weighed in water. If the relative density of steel is 7, its apparent weight is
A)
\[4\times 4\times 4\times 6g\]
done
clear
B)
\[5\times 5\times 5\times 9g\]
done
clear
C)
\[4\times 4\times 4\times 7\text{ }g\]
done
clear
D)
\[6\times 5\times 5\times 5\text{ }g\]
done
clear
View Answer play_arrow
question_answer 56) A coin is of mass 4.8 kg and radius 1 m rolling on a horizontal surface without sliding with angular velocity 600 rot/min. What is total kinetic energy of the coin?
A)
\[360\,J\]
done
clear
B)
\[1440{{\pi }^{2}}J\]
done
clear
C)
\[4000{{\pi }^{2}}J\]
done
clear
D)
\[600{{\pi }^{2}}J\]
done
clear
View Answer play_arrow
question_answer 57) 1 N/m is equal to
A)
\[1\,J{{m}^{-2}}\]
done
clear
B)
\[1\,J{{m}^{3}}\]
done
clear
C)
\[1\,J{{m}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 58) A force\[{{F}_{1}}\]of 500 N is required to push a car of mass 1000 kg slowly at constant speed on a levelled road. If a force\[{{F}_{2}}\]of 1000 N is applied, the acceleration of the car will be
A)
zero
done
clear
B)
\[1.5\text{ }m/{{s}^{2}}\]
done
clear
C)
\[1.0\text{ }m/{{s}^{2}}\]
done
clear
D)
\[0.5m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 59) A particle of mass 0.2 kg tied at the end of a spring is being rotated along a vertical circle of radius 0.5 m at critical speed of 5 m/s. The tension T in the string at the highest point of its path is
A)
8.04 N
done
clear
B)
11.96N
done
clear
C)
10 N
done
clear
D)
1.96N
done
clear
View Answer play_arrow
question_answer 60) The current gain in the common-emitter mode of a transistor is 10. The input impedance is\[20\text{ }k\Omega \]and load of resistance is\[100\text{ }k\Omega \]. The power gain is
A)
300
done
clear
B)
500
done
clear
C)
200
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 61) Volume of a gas at NTP is\[1.12\times {{10}^{-7}}c{{m}^{3}}\]. The number of molecules in it is
A)
\[3.01\times {{10}^{12}}\]
done
clear
B)
\[3.01\times {{10}^{24}}\]
done
clear
C)
\[3.01\times {{10}^{23}}\]
done
clear
D)
\[3.01\times {{10}^{20}}\]
done
clear
View Answer play_arrow
question_answer 62) 74.5 g of a metallic chloride contain 35.5 g of chlorine. The equivalent weight of the metal is
A)
19.5
done
clear
B)
35.5
done
clear
C)
39.0
done
clear
D)
78.0
done
clear
View Answer play_arrow
question_answer 63) Electron affinity is maximum for
A)
\[Cl\]
done
clear
B)
\[F\]
done
clear
C)
\[Br\]
done
clear
D)
\[I\]
done
clear
View Answer play_arrow
question_answer 64) Which of the following is paramagnetic with bond order 0.5?
A)
\[{{F}_{2}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[{{N}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 65) Metallic bond is
A)
similar to ionic bond
done
clear
B)
similar to covalent bond
done
clear
C)
neither similar to ionic nor covalent bond
done
clear
D)
formed by movement of positive charged spheres in a sea of electrons
done
clear
View Answer play_arrow
question_answer 66) The correct order of magnetic moments (spin only values in BM) among the following is (Atomic numbers:\[Mn=25,\text{ }Fe=26,\text{ }Co=27\])
A)
\[{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
B)
\[{{[MnC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[CoC{{l}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}\]
done
clear
D)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[MnC{{l}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 67) The effective electrophile in aromatic. sulphonation is
A)
\[{{H}_{2}}SO_{4}^{-}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[SO_{2}^{+}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 68) Reaction of chloroform with KOH in the presence of a primary aromatic amine is called
A)
carbylamine reaction
done
clear
B)
reduction
done
clear
C)
hydrolysis
done
clear
D)
Wurtz reaction
done
clear
View Answer play_arrow
question_answer 69) The strongest base among the following is
A)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
done
clear
View Answer play_arrow
question_answer 70) Acetamide and ethylamine can be distinguished by reacting with
A)
aqueous\[HCl\]and heat
done
clear
B)
aqueous\[NaOH\]and heat
done
clear
C)
acidified\[KMn{{O}_{4}}\]
done
clear
D)
bromine water
done
clear
View Answer play_arrow
question_answer 71) Aldol condensation would not occur in
A)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
HCHO
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 72) The most suitable reagent for the conversion of \[RC{{H}_{2}}OH\xrightarrow[{}]{{}}RCHO\] is
A)
\[KMn{{O}_{4}}\]
done
clear
B)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
C)
\[Cr{{O}_{3}}\]
done
clear
D)
PCC (pyridine chloro chromate)
done
clear
View Answer play_arrow
question_answer 73)
A)
p-benzoquinone
done
clear
B)
p-benzenediol
done
clear
C)
benzenesulphonic acid
done
clear
D)
diphenyl ether
done
clear
View Answer play_arrow
question_answer 74) The number of isomers for the compound with the molecular formula\[{{C}_{2}}BrClFI\]is
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 75) The pH of an acidic buffer mixture is
A)
6.8
done
clear
B)
7
done
clear
C)
7.5
done
clear
D)
depends upon\[{{K}_{a}}\]of the acid
done
clear
View Answer play_arrow
question_answer 76) Which of the following is optically active?
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-CHOH-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-COOH\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,=O\]
done
clear
View Answer play_arrow
question_answer 77) The \[E_{{{M}^{3+}}/M{{n}^{2+}}}^{o}\] values for\[Cr,Mn,Fe\]and\[Co\]are \[-0.41,+1.57,+0.77\] and\[+1.97V\]respectively. For which one of these metals the change in oxidation state from + 2 to + 3 is easiest?
A)
\[Cr\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Fe\]
done
clear
D)
\[Co\]
done
clear
View Answer play_arrow
question_answer 78) Crystals can be classified into basic crystal habits equal to
A)
7
done
clear
B)
4
done
clear
C)
14
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 79) According to second law of thermodynamics, a process (reaction) is spontaneous, if during the process
A)
\[\Delta {{S}_{universe}}>0\]
done
clear
B)
\[\Delta {{S}_{universe}}=0\]
done
clear
C)
\[\Delta {{H}_{system}}>0\]
done
clear
D)
\[\Delta {{S}_{universe}}=\Delta {{S}_{system}}\]
done
clear
View Answer play_arrow
question_answer 80) Insulin, a hormone, chemically is
A)
fat
done
clear
B)
steroid
done
clear
C)
protein
done
clear
D)
carbohydrate
done
clear
View Answer play_arrow
question_answer 81) These are the extraction processes of silver except
A)
as a side product in electolytic refining of copper
done
clear
B)
Parkes process in which Zn is used to extract Silver by solvent extraction from molten lead
done
clear
C)
by reaction of silver sulphide with KCN, and then reaction of soluble complex with Zn
done
clear
D)
by heating\[Na[Ag{{(CN)}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 82) The noble gas was first time discovered by
A)
Cavendish
done
clear
B)
William Ramsay
done
clear
C)
Rayleigh
done
clear
D)
Frankland
done
clear
View Answer play_arrow
question_answer 83) Which one of the following ions is colourless?
A)
\[C{{u}^{+}}\]
done
clear
B)
\[C{{o}^{2+}}\]
done
clear
C)
\[N{{i}^{2+}}\]
done
clear
D)
\[F{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 84) If the elevation in boiling point of a solution of 10 g of solute (mol. wt. =100) in 100 g of water is\[\Delta {{T}_{b}},\]the ebullioscopic constant of water is
A)
10
done
clear
B)
\[100{{T}_{b}}\]
done
clear
C)
\[\Delta {{T}_{b}}\]
done
clear
D)
\[\frac{\Delta {{T}_{b}}}{10}\]
done
clear
View Answer play_arrow
question_answer 85) Which substance is not used for preparing lyophilic sols?
A)
Starch
done
clear
B)
Gum
done
clear
C)
Gelatin
done
clear
D)
Metal sulphide
done
clear
View Answer play_arrow
question_answer 86) Excess of\[KI\]reacts with\[CuS{{O}_{4}}\]solution and then\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]solution is added to it. Which of the statements is incorrect for this reaction?
A)
\[C{{u}_{2}}{{I}_{2}}\]is formed
done
clear
B)
\[Cu{{I}_{2}}\] is formed
done
clear
C)
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]is oxidised
done
clear
D)
Evolved\[{{I}_{2}}\]is reduced
done
clear
View Answer play_arrow
question_answer 87) The change in optical rotation of freshly prepared solution of cane sugar with time is known as
A)
mutarotation
done
clear
B)
inversion
done
clear
C)
specific rotation
done
clear
D)
rotatory motion
done
clear
View Answer play_arrow
question_answer 88)
The\[{{S}_{N}}1\]reactivity of the following halides will be in the order (i) \[{{(C{{H}_{3}})}_{3}}CBr\] (ii) \[{{({{C}_{6}}{{H}_{5}})}_{2}}CHBr\] (iii) \[{{({{C}_{6}}{{H}_{5}})}_{2}}C(C{{H}_{3}})Br\] (iv) \[{{(C{{H}_{3}})}_{2}}CHBr\] (v) \[{{C}_{2}}{{H}_{5}}Br\]
A)
(ii)> (i) > (iii) > (v) > (iv)
done
clear
B)
(i)>(m)>(v)>(ii)>(iv)
done
clear
C)
(v)> (i)> (ii) > (iv) > (iii)
done
clear
D)
(iii)> (ii)> (i) > (iv) > (v)
done
clear
View Answer play_arrow
question_answer 89) Oxidation number of N is\[HN{{O}_{3}}\]is
A)
\[-3.5\]
done
clear
B)
\[+3.5\]
done
clear
C)
\[-3,+5\]
done
clear
D)
\[+5\]
done
clear
View Answer play_arrow
question_answer 90) Two gram of hydrogen diffuse from a container in 10 min. How many gram of oxygen would diffuse through the same container in the same time under similar conditions?
A)
0.5 g
done
clear
B)
4g
done
clear
C)
6 g
done
clear
D)
8 g
done
clear
View Answer play_arrow
question_answer 91)
The electrons identified by quantum number\[n\]and\[l\] (i) \[n=4,Z=l\] (ii) \[n=4,l=0\] (iii) \[n=3,l=2\] (iv) \[n=3,l=1\]
Can be placed in order of increasing energy from the lowest to highest as
A)
(iv) < (ii) < (iii) < (i)
done
clear
B)
(ii) < (i^) < (i) < (iii)
done
clear
C)
(i) < (iii) < (ii) < (iv)
done
clear
D)
(iii) < (i) < (iv) < (ii)
done
clear
View Answer play_arrow
question_answer 92) Catalytic poisons act by
A)
making the products chemically inactive
done
clear
B)
increasing the rate of the backward reaction
done
clear
C)
chemical combination with any one of the reactants
done
clear
D)
preferential adsorption on the catalyst surface
done
clear
View Answer play_arrow
question_answer 93) van der Waals equation of state is obeyed by real gases. For\[n\]moles of a real gas, the expression will be
A)
\[\left( \frac{p}{n}+\frac{na}{{{V}^{2}}} \right)\left( \frac{V}{n-b} \right)=RT\]
done
clear
B)
\[\left( P+\frac{a}{{{V}^{2}}} \right)(V-b)=nRT\]
done
clear
C)
\[\left( P+\frac{na}{{{V}^{2}}} \right)(nV-b)=nRT\]
done
clear
D)
\[\left( P+\frac{{{n}^{2}}a}{{{V}^{2}}} \right)(V-nb)=nRT\]
done
clear
View Answer play_arrow
question_answer 94) The effective atomic number of\[Cr\](atomic no. 24) in\[[Cr{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]is
A)
35
done
clear
B)
27
done
clear
C)
33
done
clear
D)
36
done
clear
View Answer play_arrow
question_answer 95) The IUPAC name of the compound having the molecular formula\[C{{l}_{3}}C.C{{H}_{2}}CHO\]is
A)
3, 3, 3-trichloropropanal
done
clear
B)
1, 1,1-trichloropropanal
done
clear
C)
2, 2, 2-trichloropropanal
done
clear
D)
chloral
done
clear
View Answer play_arrow
question_answer 96) Methyl acetate will be obtained by reacting \[C{{H}_{3}}OH\]with
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}COCl\]
done
clear
C)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
D)
All the above three
done
clear
View Answer play_arrow
question_answer 97) Rosenmund reduction is used for the preparation of
A)
aldehydes
done
clear
B)
ketones
done
clear
C)
ethers
done
clear
D)
fatty acids
done
clear
View Answer play_arrow
question_answer 98) When vapours of iso-propyl alcohol are passed over heated copper, the major product obtained is
A)
propane
done
clear
B)
propylene
done
clear
C)
acetaldehyde
done
clear
D)
acetone
done
clear
View Answer play_arrow
question_answer 99) Drying oil invariably contains
A)
linoleicacid
done
clear
B)
lauric acid
done
clear
C)
stearic acid
done
clear
D)
butyric acid
done
clear
View Answer play_arrow
question_answer 100) Iodine value is related to
A)
fats and oils
done
clear
B)
alcohols
done
clear
C)
esters
done
clear
D)
hydrocarbons
done
clear
View Answer play_arrow
question_answer 101) 4.0 g of\[NaOH\]is dissolved in 100 mL solution. The normality of the solution is
A)
0.1 N
done
clear
B)
0.5 N
done
clear
C)
4.0 N
done
clear
D)
1.0 N
done
clear
View Answer play_arrow
question_answer 102) Which one of the following is correctly matched?
A)
Emulsion-Curd
done
clear
B)
Foam-Mist
done
clear
C)
Aerosol-Smoke
done
clear
D)
Solid sol-Cake
done
clear
View Answer play_arrow
question_answer 103) Which of the following statements concerning transition elements is false?
A)
They are all metals
done
clear
B)
They easily form complex coordination compounds
done
clear
C)
Compounds containing unpaired electrons and their ions are mostly coloured
done
clear
D)
They show multiple oxidation states always differing by units of two
done
clear
View Answer play_arrow
question_answer 104) Which of the following is not attacked by hat sodium hydroxide solution?
A)
Silicon
done
clear
B)
Carbon
done
clear
C)
Tin
done
clear
D)
Lead
done
clear
View Answer play_arrow
question_answer 105) Sulphuric acid reacts with\[PC{{l}_{5}}\]to give
A)
thionyl chloride
done
clear
B)
sulphur monochloride
done
clear
C)
sulphuryl chloride
done
clear
D)
sulphur tetrachloride
done
clear
View Answer play_arrow
question_answer 106) Reduction by excess carbon at high temperature can be Successfully applied in the case of
A)
\[BeO\]and \[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[ZnO\]and \[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[CaO\]and \[C{{r}_{2}}{{O}_{3}}\]
done
clear
D)
\[BaO\]and\[{{U}_{3}}{{O}_{8}}\]
done
clear
View Answer play_arrow
question_answer 107) Which of the following has minimum\[-I-\]effect?
A)
\[-N{{O}_{2}}\]
done
clear
B)
\[-COOH\]
done
clear
C)
\[-F\]
done
clear
D)
\[-\overset{+}{\mathop{N}}\,{{R}_{3}}\]
done
clear
View Answer play_arrow
question_answer 108) The most stable carbonium ion among the following is
A)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C}}\,H{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 109) The conjugate base of\[{{H}_{2}}P{{O}_{4}}\]is
A)
\[PO_{4}^{3-}\]
done
clear
B)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[HPO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 110) The concentration of\[A{{g}^{+}}\]ion in a given saturated solution of\[AgCl\]at\[25{}^\circ C\]is \[1.06\times {{10}^{-5}}g-\] ion per litre. Thus, the solubility product of\[AgCl\]is
A)
\[0.353\times {{10}^{-10}}\]
done
clear
B)
\[0.530\times {{10}^{-10}}\]
done
clear
C)
\[1.12\times {{10}^{-10}}\]
done
clear
D)
\[2.12\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 111) Which of the following statements is true for the electrochemical Daniell cell?
A)
Electrons flow from copper electrode to zinc electrode
done
clear
B)
Current flows from zinc electrode to copper electrode
done
clear
C)
Cations move toward copper electrode
done
clear
D)
Cations move toward zinc electrode
done
clear
View Answer play_arrow
question_answer 112) Calculate the free energy change for the following reaction at 300 K. \[2CuO(s)\xrightarrow[{}]{{}}C{{u}_{2}}O(s)+\frac{1}{2}{{O}_{2}}(g)\] Given, \[\Delta H=145.6\text{ }kJ\text{ }mo{{l}^{-1}}\] and \[\Delta S=116\text{ }J{{k}^{-1}}mo{{l}^{-1}}\]
A)
\[110.8\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[221.5\text{ }kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[55.4\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[145.6\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 113) \[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]N{{O}_{2}}\]and\[[Co{{(N{{H}_{3}})}_{4}}ClN{{O}_{2}}]Cl\]exhibit which type of isomerism?
A)
Geometrical
done
clear
B)
Optical
done
clear
C)
Linkage
done
clear
D)
lonisation
done
clear
View Answer play_arrow
question_answer 114) The name of the complex\[[Pt{{(N{{H}_{3}})}_{6}}]C{{l}_{4}}\]is
A)
hexammineplatmum (IV) chloride
done
clear
B)
hexammineplatinum (II) chloride
done
clear
C)
tetrachloro hexammineplatinum (IV)
done
clear
D)
tetrachloro hexammineplatinum (II)
done
clear
View Answer play_arrow
question_answer 115) \[{{S}_{N}}1\]reaction of alkyl halides leads to
A)
retention of configuration
done
clear
B)
racemization
done
clear
C)
inversion of configuration
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 116) Phenol reacts with\[PC{{l}_{5}}\]to give mainly
A)
p-chlorophenol
done
clear
B)
chlorobenzene
done
clear
C)
o-and p-chlorophenols
done
clear
D)
triphenylphosphate
done
clear
View Answer play_arrow
question_answer 117) Rate of a reaction
A)
decreases with increase in temperature
done
clear
B)
increases with increase in temperature
done
clear
C)
may increase or decrease with increase in temperature
done
clear
D)
does not depend on temperature
done
clear
View Answer play_arrow
question_answer 118) Which one of the following reactions involves oxidation reduction?
A)
\[{{H}_{2}}+B{{r}_{2}}\xrightarrow[{}]{{}}2HBr\]
done
clear
B)
\[NaBr+HCl\xrightarrow[{}]{{}}NaCl+HBr\]
done
clear
C)
\[HBr+AgN{{O}_{3}}\xrightarrow[{}]{{}}AgBr+HN{{O}_{3}}\]
done
clear
D)
\[2NaOH+{{H}_{2}}S{{O}_{4}}\xrightarrow[{}]{{}}N{{a}_{2}}S{{O}_{4}}+2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 119) Element the electronic configuration of which is\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{3}}\] belongs to the following .group of the Periodic Table
A)
2nd
done
clear
B)
5th
done
clear
C)
3rd
done
clear
D)
7th
done
clear
View Answer play_arrow
question_answer 120) Correct electronic configuration of Cr\[(Z=24)\]is
A)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{7}}4{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}4{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{7}}4{{s}^{2}}\]
done
clear
D)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{6}}4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 121) Name given to fossil hominid of Shivalik hills in India is
A)
Ramapithecus
done
clear
B)
Australopithecus
done
clear
C)
Pithecanthropus
done
clear
D)
Neanderthalensis
done
clear
View Answer play_arrow
question_answer 122) Passive immunity can be obtained by injecting
A)
antibodies
done
clear
B)
antigen
done
clear
C)
antibiotic
done
clear
D)
vaccination
done
clear
View Answer play_arrow
question_answer 123) In pteridophytes, phloem is without
A)
sieve cells
done
clear
B)
sieve tubes
done
clear
C)
companion cells
done
clear
D)
bast fibres
done
clear
View Answer play_arrow
question_answer 124) In which plant, Calvin experimented by radioactive isotopy to discover the stable product of\[{{C}_{3}}-\]cycle?
A)
Chlorella
done
clear
B)
Cycas
done
clear
C)
Carrot
done
clear
D)
Tobacco
done
clear
View Answer play_arrow
question_answer 125) Which of the following inhibits protein synthesis by binding to\[50S\]ribosome?
A)
Tetracyclin
done
clear
B)
Streptomycin
done
clear
C)
Erythromycin
done
clear
D)
Penicillin
done
clear
View Answer play_arrow
question_answer 126) Zygospore formation occurs in
A)
Mucor
done
clear
B)
Plasmodium
done
clear
C)
Lentinum
done
clear
D)
Peziza
done
clear
View Answer play_arrow
question_answer 127) Which structure of man is similar to spiracle of cockroach?
A)
Nostril
done
clear
B)
Bronchiole
done
clear
C)
Lungs
done
clear
D)
Alveoli
done
clear
View Answer play_arrow
question_answer 128) Vagina, oesophagus, urethra contain which type of tissue?
A)
Stratified squamous epithelium
done
clear
B)
Simple squamous epithelium
done
clear
C)
Ciliated epithelium
done
clear
D)
Columnar epithelium
done
clear
View Answer play_arrow
question_answer 129) Dominant generation in bryophytes is
A)
capsule
done
clear
B)
sporophyte
done
clear
C)
gametophyte
done
clear
D)
seta
done
clear
View Answer play_arrow
question_answer 130) The radiation energy of light is converted to chemical energy and stored as
A)
AMP
done
clear
B)
ADP
done
clear
C)
ATP
done
clear
D)
APP
done
clear
View Answer play_arrow
question_answer 131) Bt cotton is resistant to
A)
insects
done
clear
B)
herbicides
done
clear
C)
salt resistant
done
clear
D)
drought resistant
done
clear
View Answer play_arrow
question_answer 132) Which pteridophyte is called as horse tail?
A)
Equisetum
done
clear
B)
Lycopodium
done
clear
C)
Marsilea
done
clear
D)
Selaginella
done
clear
View Answer play_arrow
question_answer 133) Parts of two plants were observed. Structure-A develops aerially and produces roots when comes in contact with the soil. Structure-B develops from underground part of the stem, grows obliquely becomes aerial and produces roots on its lower surface. Identify, respectively the structure of A and B.
A)
Sucker, stolen
done
clear
B)
Stolon, runner
done
clear
C)
Stolon, sucker
done
clear
D)
Runner, stolon
done
clear
View Answer play_arrow
question_answer 134) Which are of the following has epiphytic features and aerial and flattened photosynthetic roots, without formal organisation of stem and leaves?
A)
Tinospora
done
clear
B)
Trapa
done
clear
C)
Taeniophyllum
done
clear
D)
Vanda
done
clear
View Answer play_arrow
question_answer 135) Identify the correct chronological sequence periods of Mesozoic era.
A)
Carboniferous\[\to \]Permian\[\to \]Triassic\[\to \]Jurassic\[\to \]Cretaceous
done
clear
B)
Cretaceous\[\to \]Permian\[\to \]Jurassic\[\to \]Carboniferous\[\to \]Triassic
done
clear
C)
Cretaceous\[\to \]Carboniferous\[\to \]Permian \[\to \]Triassic \[\to \]Jurassic
done
clear
D)
barboniferous\[\to \]Jurassic\[\to \]Permian\[\to \] Triassic\[\to \]Cretaceous
done
clear
View Answer play_arrow
question_answer 136) In E. coli, a finished polypeptide has 162 amino acids of which the first amino acid is not a methionine compound. How many nucleotides of DNA are required to code this polypeptide?
A)
486
done
clear
B)
54
done
clear
C)
489
done
clear
D)
492
done
clear
View Answer play_arrow
question_answer 137) Which of the following substances induces mobilisation of carboxylation during germination of barley seeds
A)
Auxin
done
clear
B)
Gibberellin
done
clear
C)
Cytokinin
done
clear
D)
Abscisic acid
done
clear
View Answer play_arrow
question_answer 138) Bundle of His is a network of
A)
nerve fibres distributed in ventricles
done
clear
B)
nerve fibres found throughout the heart
done
clear
C)
muscle fibres distributed throughout the heart walls
done
clear
D)
muscle fibres found only in the ventricle wall
done
clear
View Answer play_arrow
question_answer 139) What type of cell division takes place in the functional megaspore initially in angiosperms?
A)
Homeotypic without cytokinesis
done
clear
B)
Reductional without cytokinesis
done
clear
C)
Somatic followed by cytokinesis
done
clear
D)
Meiotic followed by cytokinesis
done
clear
View Answer play_arrow
question_answer 140) In the fully organised Polygonum type of embryo sac, what is the ratio of haploid, diploid and triploid nuclei?
A)
\[3:1:3\]
done
clear
B)
\[6:0:1\]
done
clear
C)
\[6:1:0\]
done
clear
D)
\[3:2:3\]
done
clear
View Answer play_arrow
question_answer 141) The anaphase promoting complex is activated by
A)
M cdkcyclin
done
clear
B)
\[{{G}_{1}}\]cdk cyclin
done
clear
C)
S cdk cyclin
done
clear
D)
Transcription factor
done
clear
View Answer play_arrow
question_answer 142) Triticale is a hybrid formed from the members belonging to the following families
A)
Brassicaceae and Poaceae
done
clear
B)
Poaceae and Poaceae
done
clear
C)
Poaceae and Fabaceae
done
clear
D)
Alismaceae and Poaceae
done
clear
View Answer play_arrow
question_answer 143) The juice containing sodium glycocholate is released under the influence
A)
secretin
done
clear
B)
cholecystokinin
done
clear
C)
enterogastrone
done
clear
D)
enterocrinin
done
clear
View Answer play_arrow
question_answer 144) The enzyme employed for amplification of DNA during PCR is commercially obtained from
A)
Streptococcus pyrogenes
done
clear
B)
Bacillus licheniformis
done
clear
C)
Trichoderma reesi
done
clear
D)
Thermus aquaticus
done
clear
View Answer play_arrow
question_answer 145) In five kingdom system of classification of RH Whittaker, how many kingdoms contain eukaryotes?
A)
Four kingdoms
done
clear
B)
One kingdom
done
clear
C)
Two kingdoms
done
clear
D)
Three kingdoms
done
clear
View Answer play_arrow
question_answer 146) Average kilocalorie of energy needed by woman is
A)
less than man
done
clear
B)
more than man
done
clear
C)
equal to man
done
clear
D)
can not be predicted
done
clear
View Answer play_arrow
question_answer 147) Which one is not correctly matched?
A)
Mollusca -Pseudocoel
done
clear
B)
Cnidaria - Nematocyst
done
clear
C)
Annelida - Chloragogen cells
done
clear
D)
Echinodermata - Water vascular system
done
clear
View Answer play_arrow
question_answer 148) Improvement of human race is called
A)
euthenics
done
clear
B)
human heredity
done
clear
C)
human demography
done
clear
D)
eugenics
done
clear
View Answer play_arrow
question_answer 149) Blood of earthworm is
A)
red in colour, due to dissolved haemoglobin in corpuscle
done
clear
B)
red in colour, due to dissolved haemoglobin in plasma
done
clear
C)
blue in colour, due to dissolved haemocyanin in plasma
done
clear
D)
blue in colour, due to dissolved haemocyanin in corpuscles
done
clear
View Answer play_arrow
question_answer 150) Agranulocytes are
A)
lymphocytes and monocytes
done
clear
B)
eosinophils and basophils
done
clear
C)
lymphocytes and eosinophils
done
clear
D)
basophils and monocytes
done
clear
View Answer play_arrow
question_answer 151) The wish-bone or Merry though bone of birds is
A)
sternum
done
clear
B)
scapula
done
clear
C)
coracoid
done
clear
D)
clavicle
done
clear
View Answer play_arrow
question_answer 152) Symmetry in Cnidaria is
A)
radial
done
clear
B)
bilateral
done
clear
C)
pentamerous
done
clear
D)
spherical
done
clear
View Answer play_arrow
question_answer 153) TaemcLsolium is asociated with
A)
apolysis
done
clear
B)
strobilisation
done
clear
C)
premuniti,on
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 154) Sexual mode of reproduction in Protozoa
A)
anisogamy
done
clear
B)
plasmogamy
done
clear
C)
plasmotomy
done
clear
D)
schizogony
done
clear
View Answer play_arrow
question_answer 155) Which one is incorrect?
A)
Shellfish - Pisces
done
clear
B)
Silver fish - Arthopoda
done
clear
C)
Cuttle fish - Mollusca
done
clear
D)
Starfish - Echinodemata
done
clear
View Answer play_arrow
question_answer 156) Trochophore larva is found in
A)
Annelida and Mollusca
done
clear
B)
Annelida and Cnidaria
done
clear
C)
Annelida and Ctenophora
done
clear
D)
Annelida and Arthropoda
done
clear
View Answer play_arrow
question_answer 157)
Match the following lists and choose the correct option. List-I List-II A. Columnar epithelium 1. Larynx B. Ligaments 2. Eosinopaenia C. Chondnoblast 3. Elastic tissue D. Addophils 4. Urinary bladder E. Uninucleated spindle-shaped muscle fibres 5. Microvilli
A)
A- 5 B-3 C - 1 D-2 E-4
done
clear
B)
A-5 B- 1 C -3 D-2 E-4
done
clear
C)
A-1 B-5 C -3 D-2 E-4
done
clear
D)
A-5 B- 3 C -1 D-4 E-2
done
clear
View Answer play_arrow
question_answer 158)
Assertion (A): Linnaeus system of animal classification is essentially an artificial stem, yet it has become a natural system Reason (R): Similarities forming Ac basis in Linnaeus system are indicative of genetic relationship.
A)
Both (S) and (R) are true and (R) is the correct explanation to (S)
done
clear
B)
Both (S) and (R) are true, but (R) cannot explain (S)
done
clear
C)
Only (S) is true and (R) is not true
done
clear
D)
(S) is not correct and (R) cannot explain (S)
done
clear
View Answer play_arrow
question_answer 159)
The following statements are given about plant growth hormones. I. Kinetin is a degradative substance from DNA molecule. II. ABA is present, in all the plants III. Low ratio of cytpkinins to auxins favours root formation only. IV. ABA is synthesised catabolically through mevalonate pathway.
The correct combination is
A)
I and II
done
clear
B)
II and III
done
clear
C)
I and III
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 160) Which organism forms perithecia in its life
A)
Colletotrichum
done
clear
B)
Pyricularia
done
clear
C)
Helminthosporium
done
clear
D)
Sphaerotheca
done
clear
View Answer play_arrow
question_answer 161) Which phytohormone has viral inhibitory property?
A)
\[IAA\]
done
clear
B)
\[G{{A}_{3}}\]
done
clear
C)
\[ABA\]
done
clear
D)
\[2,4-D\]
done
clear
View Answer play_arrow
question_answer 162)
Study the following lists. List-I List-II A. Zacharias Janssen 1. Sexual reproduction B. Camerarius 2. Conduction of water C. Stephen Hales 3. Compound microscope D. Knoll and Ruska 4. Crystallisation of urease 5. Electron microscope
A)
A-5 B- 2 C-4 D-3
done
clear
B)
A- 3 B-1 C-2 D- 5
done
clear
C)
A- 2 B-4 C-1 D-3
done
clear
D)
A-5 B-2 C-3 D-1
done
clear
View Answer play_arrow
question_answer 163)
Assertion: Clonal selection is a method of breeding m sugarcane. Reason: sugarcane is propagated Trough suckers.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Assertion is false but Reason is true
done
clear
View Answer play_arrow
question_answer 164) By which mechanism, the salt resistant Plants can get rid off excess Na- ions to the outer side, through the roots?
A)
\[{{H}^{+}}-\]ATPase uniport system
done
clear
B)
\[N{{a}^{+}}-\]ATPase uniport system
done
clear
C)
\[{{H}^{+}}-C{{l}^{-}}\]symport system
done
clear
D)
\[N{{a}^{+}}-{{H}^{+}}\]anti port system
done
clear
View Answer play_arrow
question_answer 165) The animal as an adult secondarily acquires radial symmetry when its bilaterally symmetrical larva metamorphosis, is
A)
Polygordius
done
clear
B)
Gorgonia
done
clear
C)
Gorgonocephalus
done
clear
D)
Pila
done
clear
View Answer play_arrow
question_answer 166) The natural selection that acts against change in the form and keeps the population, constant though the time is
A)
directional
done
clear
B)
disruptive
done
clear
C)
not acting
done
clear
D)
stabilizing
done
clear
View Answer play_arrow
question_answer 167) Euploidy is best explained by
A)
exact multiple of a haploid set of chromosomes
done
clear
B)
one chromosome less than the haploid set of chromosomes
done
clear
C)
one chromosome more than the haploid set of chromosomes
done
clear
D)
one chromosome more than the diploid set of chromosomes
done
clear
View Answer play_arrow
question_answer 168) In DNA helix, cytosine is paired with guanine by
A)
three hydrogen bonds
done
clear
B)
two hydrogen bonds
done
clear
C)
single hydrogen bond
done
clear
D)
covalentbond
done
clear
View Answer play_arrow
question_answer 169) Stalk with which ovules remain attached to the placenta is called
A)
funicle
done
clear
B)
raphe
done
clear
C)
hilum
done
clear
D)
chalaza
done
clear
View Answer play_arrow
question_answer 170) Family-Gramineae is closely related to
A)
Cannaceae
done
clear
B)
Cyperaceae
done
clear
C)
Arecaceae
done
clear
D)
Apicaceae
done
clear
View Answer play_arrow
question_answer 171) Edward, Patau and Downs syndromes are
A)
change in autosomes
done
clear
B)
changes in sex chromosomes
done
clear
C)
mutation due to malnutrition
done
clear
D)
both change in sex chromosome and autosomes
done
clear
View Answer play_arrow
question_answer 172) Biosphere reserve programme started in India?
A)
1986
done
clear
B)
1984
done
clear
C)
1982
done
clear
D)
1988
done
clear
View Answer play_arrow
question_answer 173) Peroxispmes are found in
A)
bundle sheath
done
clear
B)
endosperm
done
clear
C)
mesophyll cells
done
clear
D)
vascular bundles
done
clear
View Answer play_arrow
question_answer 174) Low calorie and low cholesterol is found in
A)
soyabean oil
done
clear
B)
peanut oil
done
clear
C)
sesame oil
done
clear
D)
sunflower oil
done
clear
View Answer play_arrow
question_answer 175) Algae, which form motile colony, is
A)
Volvox
done
clear
B)
Nostoc
done
clear
C)
Spirogyra
done
clear
D)
Chlamydomonas
done
clear
View Answer play_arrow
question_answer 176) Short day plant is
A)
Xanthium
done
clear
B)
Pisum
done
clear
C)
Cucumis
done
clear
D)
Avena
done
clear
View Answer play_arrow
question_answer 177) A patient of diabetes mellitus excretes glucose in urine even when he is kept in a carbohydrate free diet. It is because
A)
fats are catabolised to form glucose
done
clear
B)
amino acids are catabolised in liver
done
clear
C)
amino acids are discharged in blood stream from liver
done
clear
D)
glycogen from muscles are released in the blood stream
done
clear
View Answer play_arrow
question_answer 178) Diphtheria is characterised by
A)
suffocation
done
clear
B)
hydrophobia
done
clear
C)
dehydration
done
clear
D)
gum bleeding
done
clear
View Answer play_arrow
question_answer 179) The triploid number of chromosomes of the first taxon is ten times more than the haploid number of chromosomes of the second taxon while the diploid number of the third taxon is six time more than the haploid number of the fourth taxon. Which one of the following shows the ascending order of the number of chromosomes in their respective endosperm?
A)
Oryza-Allium-Saccharum-Nicotiana
done
clear
B)
Allium-Oryza-Nicotmna-Saccharum
done
clear
C)
Nicotiana-Saccharum-Oryza-Allium
done
clear
D)
Saccharum-Oryza-Nicotiana-Allium
done
clear
View Answer play_arrow
question_answer 180)
Match the following lists. List-I List-II A. Basophils 1. Phagocytosis B. Neutrophils 2. Inflammation C. Plasma cells 3. Blood clotting D. Thrombocytes 4. Antibodies
A)
A- 2 B- 1 C-4 D-3
done
clear
B)
A-2 B-1 C-3 D-4
done
clear
C)
A-1 B-2 C-4 D-3
done
clear
D)
A-4 B-1 C-2 D-3
done
clear
View Answer play_arrow
question_answer 181)
Assertion: Libriform fibres are true fibres. Reason: Libriform fibre develop from non-functional tracheids by reduction.
A)
Both Assertion and Reason are true and Reason is the explanation of Assertion
done
clear
B)
Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Assertion is false but Reason is true
done
clear
View Answer play_arrow
question_answer 182)
The following are the branches of dorsal aorta. I. Intercostal II. Phrenic III. Coeliac IV. Anterior mesenteric V. Posterior mesenteric
Of these, which set of arteries supply the blood to the glands of digestive system?
A)
I and II
done
clear
B)
III and IV
done
clear
C)
IV and V
done
clear
D)
II and III
done
clear
View Answer play_arrow
question_answer 183) The water soluble protein associated with silk thread is
A)
fibroin
done
clear
B)
serecin
done
clear
C)
chitin
done
clear
D)
mucin
done
clear
View Answer play_arrow
question_answer 184)
Consider the following sentences I. Dentition is heterodont. II. Canines are poorly developed. III. Incisors are chisel like and poorly developed. IV. Herbivorous and diastema is present. V. The dental formula is,\[I\text{ }2/1;\text{ }C\text{ }0/0;\] Pm 3/2; M 3/3.
Which of the above are true for Oryctolagus?
A)
I, II, IV
done
clear
B)
I, IV, V
done
clear
C)
I, II, IV, V
done
clear
D)
II, IV, V
done
clear
View Answer play_arrow
question_answer 185) If sexual reproduction takes place between the filaments of Rhizopus of different strains, one with 80 nuclei and another with 24 nuclei, what would be the total number of spores of different strains put together?
A)
24
done
clear
B)
48
done
clear
C)
96
done
clear
D)
114
done
clear
View Answer play_arrow
question_answer 186) Which of the following is indicative of the term alburnum?
A)
Spring wood
done
clear
B)
Autumn wood
done
clear
C)
Heart wood
done
clear
D)
Sap wood
done
clear
View Answer play_arrow
question_answer 187) The cranial nerve that goes to the external rectus muscle is
A)
II
done
clear
B)
III
done
clear
C)
VII
done
clear
D)
VI
done
clear
View Answer play_arrow
question_answer 188) A student collected a hydrophyte with swollen and with a single vascular bundle in the root. The plant which he collected was
A)
Jussiaea
done
clear
B)
Trapa
done
clear
C)
Ceratophyllum
done
clear
D)
Potamogeton
done
clear
View Answer play_arrow
question_answer 189) A snake is identified to be having large hexagonal vertebral and the dorsal surface bluish with narrow white streaks, it is
A)
Echis carinata
done
clear
B)
Bungarus coeruleus
done
clear
C)
Vipera russelli
done
clear
D)
Hemibungarus
done
clear
View Answer play_arrow
question_answer 190) The raw material obtained from which one of the following plants is used in paper making?
A)
Jerusalem artichoke
done
clear
B)
Oryza sativa
done
clear
C)
Sorghum vulgare
done
clear
D)
Butea monosperma
done
clear
View Answer play_arrow
question_answer 191) Binomial nomenclatures is first mentioned in the book
A)
Systema Naturae
done
clear
B)
Historia Animalium
done
clear
C)
Historia Plantarum
done
clear
D)
Philosophie Zoologique
done
clear
View Answer play_arrow
question_answer 192) Hugo de Vries observed mutation in
A)
Pisum sativum
done
clear
B)
Arabidopsis thaliana
done
clear
C)
Oenothera lamarckiana
done
clear
D)
Datura stramonium
done
clear
View Answer play_arrow
question_answer 193) Bacterium which reduces nitrates in soil to nitrogen is
A)
Nitrosomonas
done
clear
B)
Pseudomonas
done
clear
C)
Rhizobium
done
clear
D)
Clostridium
done
clear
View Answer play_arrow
question_answer 194) If position of ovary is below sepals, petals, stamens, the flower is called.
A)
epigynous
done
clear
B)
perigynous
done
clear
C)
mesogynous
done
clear
D)
metagynous
done
clear
View Answer play_arrow
question_answer 195) Study of ticks and mites is
A)
Acarology
done
clear
B)
Entomology
done
clear
C)
Malacology
done
clear
D)
Carcinology
done
clear
View Answer play_arrow
question_answer 196) Asiatic lion (Panthera leo persicd) is now
A)
endangered
done
clear
B)
extinct in wild
done
clear
C)
vulnerable
done
clear
D)
critically endangered
done
clear
View Answer play_arrow
question_answer 197) Muscles which bend the joint
A)
flexor
done
clear
B)
extensor
done
clear
C)
involution
done
clear
D)
twitch
done
clear
View Answer play_arrow
question_answer 198) Fungal flagellum originates from
A)
dictyosomes
done
clear
B)
kinetosomes
done
clear
C)
glyoxysomes
done
clear
D)
oxysomes
done
clear
View Answer play_arrow
question_answer 199) Sucking roots are present in the plant
A)
betel
done
clear
B)
Cuscuta
done
clear
C)
Mangifera
done
clear
D)
Solanum
done
clear
View Answer play_arrow
question_answer 200) In acid rain,\[S{{O}_{2}}\]accounts for
A)
70%
done
clear
B)
100%
done
clear
C)
50%
done
clear
D)
30%
done
clear
View Answer play_arrow
question_answer 201) Which one of the following statements is true?
A)
An engine-driver is cleverer than Plato or Socrates
done
clear
B)
Plato .or Socrates is in no way inferior to the engine-driver
done
clear
C)
Plato and Socrates surpassed the engine-driver in every respect
done
clear
D)
The engine-driver cannot be compared to Plato or Aristotle
done
clear
View Answer play_arrow
question_answer 202) In this passage, the author mentions Plato and/or Socrates to emphasise that
A)
they are/were men of great scholarship
done
clear
B)
people as individuals in the modem age are not more advanced than their predecessors
done
clear
C)
the engine is a better mode of locomotion than Platos chariot
done
clear
D)
Plato and Aristotle had greater respect for learning
done
clear
View Answer play_arrow
question_answer 203) According to the author, the present age is far more advanced than
A)
all the previous ages in some respect
done
clear
B)
the age of Socrates and Aristotle in some respects
done
clear
C)
some of the previous ages in all respects
done
clear
D)
all the previous ages in all respects
done
clear
View Answer play_arrow
question_answer 204) Many of us make use of machines
A)
with very little knowledge of their mechanism
done
clear
B)
without any knowledge of their historical significance
done
clear
C)
with full knowing of their genesis
done
clear
D)
without knowing how they were invented
done
clear
View Answer play_arrow
question_answer 205) People today are very proud because they live
A)
in a philosophically advanced age
done
clear
B)
in a materially advanced age
done
clear
C)
in a scientifically advanced age
done
clear
D)
in a spiritually advanced age
done
clear
View Answer play_arrow
question_answer 206) Directions: In each of the following questions, choose the alternative which best expresses the meaning of the word given in capital letters. RARE
A)
common
done
clear
B)
usual
done
clear
C)
scarce
done
clear
D)
few
done
clear
View Answer play_arrow
question_answer 207) Directions: In each of the following questions, choose the alternative which best expresses the meaning of the word given in capital letters. CRAVEN
A)
greedy
done
clear
B)
cowardly
done
clear
C)
flattering
done
clear
D)
restless
done
clear
View Answer play_arrow
question_answer 208) Directions: In each of the following questions, choose the alternative which best expresses the meaning of the word given in capital letters. VICARIOUS
A)
ambitious
done
clear
B)
not experienced personally
done
clear
C)
nostalgic
done
clear
D)
vindictive
done
clear
View Answer play_arrow
question_answer 209) Directions: In each of the following questions, choose the alternative which best expresses the meaning of the word given in capital letters. TRANSITION
A)
position
done
clear
B)
translation
done
clear
C)
change
done
clear
D)
movement
done
clear
View Answer play_arrow
question_answer 210) Directions: In each of the following questions, find out the part which has an error. If there is no error, your answer is [d].
A)
The train come/
done
clear
B)
at 2 Oclock/
done
clear
C)
in the next morning./
done
clear
D)
No error/
done
clear
View Answer play_arrow
question_answer 211) Directions: In each of the following questions, find out the part which has an error. If there is no error, your answer is [d].
A)
I have not been to/
done
clear
B)
New york before/
done
clear
C)
and neither my sister/
done
clear
D)
No error/
done
clear
View Answer play_arrow
question_answer 212) Directions: In each of the following questions, find out the part which has an error. If there is no error, your answer is [d].
A)
A major contribution of Mathura sculptors/
done
clear
B)
of that period were the creation and popularization/
done
clear
C)
of the Buddhas image in human form./
done
clear
D)
No error/
done
clear
View Answer play_arrow
question_answer 213) Directions: In each of the following questions, find out the part which has an error. If there is no error, your answer is [d].
A)
I had hoped that/
done
clear
B)
I would see you the other day/
done
clear
C)
but unfortunately/
done
clear
D)
I fell ill./
done
clear
View Answer play_arrow
question_answer 214) An allowance paid to wife on divorce
A)
Alimony
done
clear
B)
Bigamy
done
clear
C)
Celibacy
done
clear
D)
Matrimony
done
clear
View Answer play_arrow
question_answer 215) The study of human races
A)
Botany
done
clear
B)
Biology
done
clear
C)
Ethnology
done
clear
D)
Geology
done
clear
View Answer play_arrow
question_answer 216) Ones body can be kept healthy by adopting breathing programmes that use the respiratory system to its maximum potential, letting in.....216....oxygen......217.... the bod as possible while removing as much...218. carbon dioxide as possible. The mos effective is the total Breath Control. Practic it.... 219... it becomes second nature. When that happens, you....220... find that you an less tired.
A)
as much
done
clear
B)
as little as
done
clear
C)
into
done
clear
D)
onto
done
clear
View Answer play_arrow
question_answer 217) Ones body can be kept healthy by adopting breathing programmes that use the respiratory system to its maximum potential, letting in.....216....oxygen......217.... the bod as possible while removing as much...218. carbon dioxide as possible. The mos effective is the total Breath Control. Practic it.... 219... it becomes second nature. When that happens, you....220... find that you an less tired.
A)
in
done
clear
B)
through
done
clear
C)
into
done
clear
D)
onto
done
clear
View Answer play_arrow
question_answer 218) Ones body can be kept healthy by adopting breathing programmes that use the respiratory system to its maximum potential, letting in.....216....oxygen......217.... the bod as possible while removing as much...218. carbon dioxide as possible. The mos effective is the total Breath Control. Practic it.... 219... it becomes second nature. When that happens, you....220... find that you an less tired.
A)
useful
done
clear
B)
necessary
done
clear
C)
waste
done
clear
D)
need
done
clear
View Answer play_arrow
question_answer 219) Ones body can be kept healthy by adopting breathing programmes that use the respiratory system to its maximum potential, letting in.....216....oxygen......217.... the bod as possible while removing as much...218. carbon dioxide as possible. The mos effective is the total Breath Control. Practic it.... 219... it becomes second nature. When that happens, you....220... find that you an less tired.
A)
as soon as
done
clear
B)
until
done
clear
C)
after
done
clear
D)
till
done
clear
View Answer play_arrow
question_answer 220) Ones body can be kept healthy by adopting breathing programmes that use the respiratory system to its maximum potential, letting in.....216....oxygen......217.... the bod as possible while removing as much...218. carbon dioxide as possible. The mos effective is the total Breath Control. Practic it.... 219... it becomes second nature. When that happens, you....220... find that you an less tired.
A)
can
done
clear
B)
should
done
clear
C)
must
done
clear
D)
ought
done
clear
View Answer play_arrow
question_answer 221) Directions: In the following questions, choose the option which shows common feature in the relationship gives in each question. Nissan : Toyota : Isuzu
A)
These are cities in Japan
done
clear
B)
These are ports in Japan
done
clear
C)
These are cars from Japan
done
clear
D)
These are tele-programmes
done
clear
View Answer play_arrow
question_answer 222) Directions: In the following questions, choose the option which shows common feature in the relationship gives in each question. Yenisei: Orinoco : Makenzie
A)
These are small round hills
done
clear
B)
These are sea ports
done
clear
C)
These are names of rivers
done
clear
D)
These are rich agricultural lands
done
clear
View Answer play_arrow
question_answer 223) Directions: In the following questions, choose the option which shows common feature in the relationship gives in each question. Vinci: Angelo : Raphael
A)
They were Italian engineers
done
clear
B)
They were European painters
done
clear
C)
They were dictators
done
clear
D)
They were famous politicians
done
clear
View Answer play_arrow
question_answer 224) Directions: In the following questions, choose the option which shows common feature in the relationship gives in each question. Patna : Mumbai: Dispur
A)
Cochin : Trombay : Chennai
done
clear
B)
Delhi: Udaipur : Jammu and Kashmir
done
clear
C)
Coal: Ebony : Soot
done
clear
D)
Botany : Zoology : Mathematics
done
clear
View Answer play_arrow
question_answer 225) Directions: In the following questions, choose the option which shows common feature in the relationship gives in each question. Theta : Phi: Omega
A)
These are Latin alphabets
done
clear
B)
These are signs of algebra
done
clear
C)
These are Greek letters
done
clear
D)
These are used in physical derivations
done
clear
View Answer play_arrow
question_answer 226) Directions: In each of the following questions, there occurs a specific relations. Fill the vacant space according to that relation. Homicide : Human : : Fratricide :?
A)
Mother
done
clear
B)
Father
done
clear
C)
Brother
done
clear
D)
Enemy
done
clear
View Answer play_arrow
question_answer 227) Directions: In each of the following questions, there occurs a specific relations. Fill the vacant space according to that relation. Horse : Jockey : : Car :?
A)
Mechanic
done
clear
B)
Chauffer
done
clear
C)
Steering
done
clear
D)
Brake
done
clear
View Answer play_arrow
question_answer 228) Directions: In each of the following questions, there occurs a specific relations. Fill the vacant space according to that relation. Wool: Sheep : : Mohair :?
A)
Deer
done
clear
B)
Goat
done
clear
C)
Bear
done
clear
D)
Camel
done
clear
View Answer play_arrow
question_answer 229) Directions: In each of the following questions, there occurs a specific relations. Fill the vacant space according to that relation. Cunning : Fox : : Timid :?
A)
Horse
done
clear
B)
Ant
done
clear
C)
Ass
done
clear
D)
Panther
done
clear
View Answer play_arrow
question_answer 230) Directions: In each of the following questions, there occurs a specific relations. Fill the vacant space according to that relation. Mars : Planet: Moon :?
A)
Earth
done
clear
B)
Sun
done
clear
C)
Saturn
done
clear
D)
Satellite
done
clear
View Answer play_arrow
question_answer 231) Who was the writer of the book Arthashastra?
A)
Vatsyayana
done
clear
B)
Kautitya
done
clear
C)
Painin
done
clear
D)
Kalidas
done
clear
View Answer play_arrow
question_answer 232) 19thCommonwealth Game was held in India at
A)
New Delhi
done
clear
B)
Hyderabad
done
clear
C)
Bengaluru
done
clear
D)
Chennai
done
clear
View Answer play_arrow
question_answer 233) The headquarter of SAARC is located at
A)
New Delhi
done
clear
B)
Islamabad
done
clear
C)
Kathmandu
done
clear
D)
Colombo
done
clear
View Answer play_arrow
question_answer 234) The first Indian woman who became IPS officer, is
A)
Santosh Yadav
done
clear
B)
Arati Saha
done
clear
C)
ReetaFaria
done
clear
D)
KiranBedi
done
clear
View Answer play_arrow
question_answer 235) Who among the following Sikh Gurus started the Gurumukhi Script?
A)
GuruArjun
done
clear
B)
Guru Ramdas
done
clear
C)
Guru Tegh Bahadur
done
clear
D)
Guru Angad
done
clear
View Answer play_arrow
question_answer 236) Which was the first metal used by Man?
A)
Copper
done
clear
B)
Silver
done
clear
C)
Bronze
done
clear
D)
Brass
done
clear
View Answer play_arrow
question_answer 237) Who founded the Banaras Hindu University?
A)
Mahatma Gandhi
done
clear
B)
Madan Mohan Malviya
done
clear
C)
Jawaharlal Nehru
done
clear
D)
Motilal Nehru
done
clear
View Answer play_arrow
question_answer 238) Niagara falls are in
A)
Australia
done
clear
B)
UK
done
clear
C)
South Africa
done
clear
D)
USA
done
clear
View Answer play_arrow
question_answer 239) Folk painting Madhubanf is famous in
A)
West Bengal
done
clear
B)
Orissa
done
clear
C)
Bihar
done
clear
D)
Assam
done
clear
View Answer play_arrow
question_answer 240) Who is the proponent and propagandist of the Art of Living?
A)
Mahesh Yogi
done
clear
B)
Ram Dev
done
clear
C)
Sri Ravi Shankar
done
clear
D)
Chinmayananda
done
clear
View Answer play_arrow