A) 32 mL
B) 24 mL
C) 16 mL
D) 8 mL
Correct Answer: A
Solution :
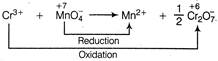 Equivalents of\[C{{r}^{3+}}=3\times \]moles of\[C{{r}^{3+}}\] Equivalents of \[MnO_{4}^{-}=5\times \]moles of\[MnO_{4}^{-}\] Amount of \[C{{r}^{3+}}=0.125\times V\,\]milli mol \[=0.125\times V\times 3\,\]miliequiv. Amount of\[MnO_{4}^{-}=0.200\times 12.00\times 5\]milliequiv \[\therefore \]\[0.125\times V\times 3=0.200\times 12.00\times 5\] \[V=32.0\,mL\]
Equivalents of\[C{{r}^{3+}}=3\times \]moles of\[C{{r}^{3+}}\] Equivalents of \[MnO_{4}^{-}=5\times \]moles of\[MnO_{4}^{-}\] Amount of \[C{{r}^{3+}}=0.125\times V\,\]milli mol \[=0.125\times V\times 3\,\]miliequiv. Amount of\[MnO_{4}^{-}=0.200\times 12.00\times 5\]milliequiv \[\therefore \]\[0.125\times V\times 3=0.200\times 12.00\times 5\] \[V=32.0\,mL\]
You need to login to perform this action.
You will be redirected in
3 sec
