A) \[{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}\]
B) \[{{[MnC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[CoC{{l}_{4}}]}^{2-}}\]
C) \[{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}\]
D) \[{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[MnC{{l}_{4}}]}^{2-}}\]
Correct Answer: A
Solution :
As the number of unpaired electrons in the central atom increases, magnetic moment of the complex increases. In\[{{[MnC{{l}_{4}}]}^{2-}}=[Ar]\]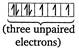 In \[{{[Fe{{(CN)}_{6}}]}^{4-}}=[Ar]\]
In \[{{[Fe{{(CN)}_{6}}]}^{4-}}=[Ar]\] 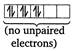 \[\therefore \]The order of magnetic moments is \[{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}\]
\[\therefore \]The order of magnetic moments is \[{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}\]
You need to login to perform this action.
You will be redirected in
3 sec
