A) zero
B) 0.88
C) 1.33
D) 2
Correct Answer: C
Solution :
We know that carbonate ion has following resonating structures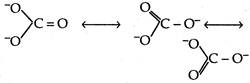 Border order \[=\frac{total\text{ }number\text{ }of\text{ }bonds\text{ }between\text{ }atoms}{total\text{ }number\text{ }of\text{ }resonating\text{ }structures}\] \[=\frac{1+1+2}{3}=\frac{4}{3}\] \[=1.33\]
Border order \[=\frac{total\text{ }number\text{ }of\text{ }bonds\text{ }between\text{ }atoms}{total\text{ }number\text{ }of\text{ }resonating\text{ }structures}\] \[=\frac{1+1+2}{3}=\frac{4}{3}\] \[=1.33\]
You need to login to perform this action.
You will be redirected in
3 sec
