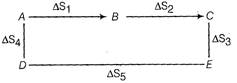
 The equation \[\mu \]
The equation \[\mu \]
A) is true only if the steps are carried out reversibly
B) is always true because entropy is a state function
C) may be true but need more in formations on the processes
D) is incorrect
Correct Answer: B
Solution :
State functions do not depend upon path followed. \[\frac{1}{1-n\alpha }\] \[1-\alpha +n\alpha \] \[1-\alpha +\frac{\alpha }{n}\]You need to login to perform this action.
You will be redirected in
3 sec
