A) \[f(x)=\frac{x}{\sqrt{1+{{x}^{2}}}},\]
B) \[\frac{x}{\sqrt{1+{{x}^{2}}}}\]
C) \[\frac{x}{\sqrt{1+3{{x}^{2}}}}\]
D) \[f(x)=\frac{{{a}^{x}}+{{a}^{-x}}}{2}\]
Correct Answer: B , C
Solution :
Initially for container A, \[{{V}_{0}}\] For container B, \[{{T}_{0}}\] \[{{P}_{0}}\]\[2{{T}_{0}}\] Total number of moles \[2{{T}_{0}}\]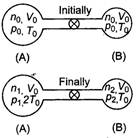 Since, even on heating the total number of moles is conserved Hence, \[p=2{{p}_{0}}\] ?(i) If p be the common pressure, then For container A, \[p=\frac{4}{3}{{p}_{0}}\]\[n=\frac{2}{3}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\]\[n=\frac{3}{2}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\] For container A,\[\frac{pV}{RT}\] \[2:\pi \]\[\pi :2\] Substituting the value of \[\lambda \] and \[\lambda /2\]in Eq. (i) we get\[\lambda /3\] Number of moles in container A (at temperature \[\lambda /4\]) \[\lambda /9\] \[{{10}^{-10}}W/{{m}^{2}}.\]
Since, even on heating the total number of moles is conserved Hence, \[p=2{{p}_{0}}\] ?(i) If p be the common pressure, then For container A, \[p=\frac{4}{3}{{p}_{0}}\]\[n=\frac{2}{3}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\]\[n=\frac{3}{2}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\] For container A,\[\frac{pV}{RT}\] \[2:\pi \]\[\pi :2\] Substituting the value of \[\lambda \] and \[\lambda /2\]in Eq. (i) we get\[\lambda /3\] Number of moles in container A (at temperature \[\lambda /4\]) \[\lambda /9\] \[{{10}^{-10}}W/{{m}^{2}}.\]
Solution :
Initially for container A, \[{{V}_{0}}\] For container B, \[{{T}_{0}}\] \[{{P}_{0}}\]\[2{{T}_{0}}\] Total number of moles \[2{{T}_{0}}\]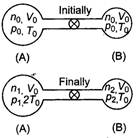 Since, even on heating the total number of moles is conserved Hence, \[p=2{{p}_{0}}\] ?(i) If p be the common pressure, then For container A, \[p=\frac{4}{3}{{p}_{0}}\]\[n=\frac{2}{3}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\]\[n=\frac{3}{2}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\] For container A,\[\frac{pV}{RT}\] \[2:\pi \]\[\pi :2\] Substituting the value of \[\lambda \] and \[\lambda /2\]in Eq. (i) we get\[\lambda /3\] Number of moles in container A (at temperature \[\lambda /4\]) \[\lambda /9\] \[{{10}^{-10}}W/{{m}^{2}}.\]
Since, even on heating the total number of moles is conserved Hence, \[p=2{{p}_{0}}\] ?(i) If p be the common pressure, then For container A, \[p=\frac{4}{3}{{p}_{0}}\]\[n=\frac{2}{3}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\]\[n=\frac{3}{2}\frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}}\] For container A,\[\frac{pV}{RT}\] \[2:\pi \]\[\pi :2\] Substituting the value of \[\lambda \] and \[\lambda /2\]in Eq. (i) we get\[\lambda /3\] Number of moles in container A (at temperature \[\lambda /4\]) \[\lambda /9\] \[{{10}^{-10}}W/{{m}^{2}}.\]
You need to login to perform this action.
You will be redirected in
3 sec
