A) \[Mg+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}MgO\]
B) \[2Ag+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}A{{g}_{2}}O\]
C) \[CO+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}\]
D) All of the above
Correct Answer: D
Solution :
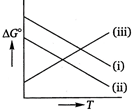 Reaction (i)\[M(s)+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}MO(s)\] [where,\[M=\]highly reactive metal] Reaction (ii)\[CO(g)+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}(g)\] Reaction (iii)\[C(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\] \[\Delta {{G}^{o}}\,\,vs\,\,T\]plot in the Ellinghams diagram slopes downwards for the reaction (I) and reaction (II). For reaction (III)\[\Delta {{G}^{o}}\,\,vs\,\,T\]in the diagram slopes upwards.
Reaction (i)\[M(s)+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}MO(s)\] [where,\[M=\]highly reactive metal] Reaction (ii)\[CO(g)+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}(g)\] Reaction (iii)\[C(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\] \[\Delta {{G}^{o}}\,\,vs\,\,T\]plot in the Ellinghams diagram slopes downwards for the reaction (I) and reaction (II). For reaction (III)\[\Delta {{G}^{o}}\,\,vs\,\,T\]in the diagram slopes upwards.
You need to login to perform this action.
You will be redirected in
3 sec
