question_answer 1) Minimum deviation is observed with a prism having angle of prism A, angle of deviation \[\delta ,\] angle of incident \[i\] and angle of emergence e, then we have generally:
A)
\[i=e\]
done
clear
B)
\[i<e\]
done
clear
C)
\[i>e\]
done
clear
D)
\[i=e=0\]
done
clear
View Answer play_arrow
question_answer 2) It takes two seconds to heat water to boiling point using a 10 m length heater coil. What time will it take for the same using 5 m of heater coil connected to the same source:
A)
2 s
done
clear
B)
3 s
done
clear
C)
1 s
done
clear
D)
10.5 s
done
clear
View Answer play_arrow
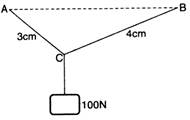
question_answer 3) A and B are two nails on a horizontal line. AC, BC are two uniform strings from which a weight of 100 N is suspended. The tension of AC is: (in N):
A)
100 N
done
clear
B)
80 N
done
clear
C)
60 N
done
clear
D)
50 N
done
clear
View Answer play_arrow
question_answer 4) An accurate and reliable audio oscillator is used to standardize a tuning fork marked as 512 Hz. When the oscillator reading is 514 two beats are heard per second. When the oscillator reading is 510, the beat frequency is 6 Hz. The frequency of the tuning fork is:
A)
518
done
clear
B)
516
done
clear
C)
506
done
clear
D)
510
done
clear
View Answer play_arrow
question_answer 5) If yellow light in the Youngs double slit experiment is replaced by red light the fringe width will:
A)
increase
done
clear
B)
decrease
done
clear
C)
remain unaffected
done
clear
D)
first increase and then decrease
done
clear
View Answer play_arrow
question_answer 6) The displacement y of a particle executing periodical motion is given by \[y=4\,\cos {{\,}^{2}}\left( \frac{t}{2} \right)\sin \,(1000t)\] This expression may be considered to be a result of the superposition of independent harmonic motions:
A)
five
done
clear
B)
four
done
clear
C)
three
done
clear
D)
two
done
clear
View Answer play_arrow
question_answer 7) A sound wave of wavelength \[\lambda \] travels towards the right horizontally with a velocity \[\upsilon ,\]. It strikes and reflects from a vertical plane surface, travelling at a speed \[\upsilon \] towards the left. The number of positive crests striking in a time interval of these second on the wall is:
A)
\[\frac{(\upsilon -\upsilon )}{3\lambda }\]
done
clear
B)
\[\frac{3(\upsilon +\upsilon )}{\lambda }\]
done
clear
C)
\[\frac{(\upsilon +\upsilon )}{3\lambda }\]
done
clear
D)
\[\frac{3(\upsilon -\upsilon )}{\lambda }\]
done
clear
View Answer play_arrow
question_answer 8) A ray of light is incident at an angle of \[54{}^\circ \] on an equilateral prism, so that it suffers minimum deviation. The angle of minimum deviation is:
A)
\[54{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[48{}^\circ \]
done
clear
D)
\[50{}^\circ \]
done
clear
View Answer play_arrow
question_answer 9) A body is projected at an angle \[\alpha \] from a point O. Its angular momentum will be zero at:
A)
the point where it touches ground
done
clear
B)
another point on trajectory
done
clear
C)
starting point
done
clear
D)
at the maximum height
done
clear
View Answer play_arrow
question_answer 10) Focal length of a convex lens will be maximum for:
A)
Red light
done
clear
B)
yellow light
done
clear
C)
Green light
done
clear
D)
blue light
done
clear
View Answer play_arrow
question_answer 11) One end of a copper rod of length 1.0 m and area of cross-section \[{{10}^{-3}}{{m}^{2}}\] is immersed in boiling water and the other end in ice. If the coefficient of thermal conductivity of copper is \[92\text{ }cal\text{/}m-s\text{ }{}^\circ C\] and the latent heat of ice is \[8\times {{10}^{4}}cal\text{/}kg,\] then the amount of ice which will melt in one minute is:
A)
\[8\times {{10}^{-3}}kg\]
done
clear
B)
\[9.2\times {{10}^{-3}}kg\]
done
clear
C)
\[5.4\times {{10}^{-3}}kg\]
done
clear
D)
\[6.9\times {{10}^{-3}}kg\]
done
clear
View Answer play_arrow
question_answer 12) Which of the following is a paramagnetic group?
A)
aluminium water and manganese
done
clear
B)
oxygen manganese and aluminium
done
clear
C)
only water and manganese
done
clear
D)
magnesium aluminium and copper
done
clear
View Answer play_arrow
question_answer 13) Two plane mirrors are inclined to each other ray of light incident on one face at \[30{}^\circ \] after reflection from the second retraces the path. What is the angle between the mirrors?
A)
\[40{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[20{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 14) A body projected with an initial velocity horizontally has kinetic energy K. If it is projected at an angle \[45{}^\circ \] the initial kinetic energy will be:
A)
0.7 K
done
clear
B)
1.41 K
done
clear
C)
2K
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 15) The amplification factor of a triode depends on the:
A)
filament voltage
done
clear
B)
plate voltage
done
clear
C)
grid voltage
done
clear
D)
relative position of cathode grid and plate
done
clear
View Answer play_arrow
question_answer 16) The resistivity of metal increases with increase in temperature because:
A)
the speed of the electrons in the metal ions decreases
done
clear
B)
the average distance between the metal ions increases
done
clear
C)
the concentration of conduction electrons increases
done
clear
D)
the concentration of conduction electrons decreases
done
clear
View Answer play_arrow
question_answer 17) If the distance of the far point for a myopia pa dent is doubled, the focal length of the lens required to cure it will become:
A)
double
done
clear
B)
half
done
clear
C)
the same but a concave lens
done
clear
D)
the same but a convex lens
done
clear
View Answer play_arrow
question_answer 18) Two solid spheres of iron have radii in the ratio 1 : 2, their moments of inertia will be in the ratio:
A)
1 : 32
done
clear
B)
1 : 16
done
clear
C)
1 : 8
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 19) In a deflection magnetometer, the external magnet should be replaced:
A)
always in the direction of resultant of earth
done
clear
B)
always with its axis along E-W
done
clear
C)
along N-S in tan A position
done
clear
D)
always along N-S
done
clear
View Answer play_arrow
question_answer 20) Glaciers melt:
A)
first at the bottom due to decrease in pressure
done
clear
B)
first at the top due to increase in pressure
done
clear
C)
first at the bottom due to increase in pressure
done
clear
D)
first at the top due to decrease in pressure
done
clear
View Answer play_arrow
question_answer 21) S is a source. Intensity of light received at Q is P. The intensity of light received at \[{{Q}_{1}}\] as shown in figure is:
A)
0.33 P
done
clear
B)
0.44 P
done
clear
C)
0.25 P
done
clear
D)
0.5 P
done
clear
View Answer play_arrow
question_answer 22) Two identical gas tanks are placed on the two pans of beam balance. One tank is empty and open to the atmosphere. The second tank is evacuated and then filled with helium unit the two tanks balance. The pressure of helium must be:
A)
1 atm
done
clear
B)
4 atm
done
clear
C)
15 atm
done
clear
D)
7.5 atm
done
clear
View Answer play_arrow
question_answer 23) In figure (below) the charge on the \[1.5\,\mu F\] capacitor is:
A)
60 \[\,\mu C\]
done
clear
B)
90 \[\,\mu C\]
done
clear
C)
120 \[\,\mu C\]
done
clear
D)
30 \[\,\mu C\]
done
clear
View Answer play_arrow
question_answer 24) Which of the following wave has the maximum intensity:
A)
Wavelength Frequency \[4\times {{10}^{-5}}\text{m}\] 100
done
clear
B)
\[4\times {{10}^{-5}}\text{m}\] 200
done
clear
C)
\[4\times {{10}^{-5}}\text{m}\] 500
done
clear
D)
\[4\times {{10}^{-5}}\text{m}\] 5000
done
clear
View Answer play_arrow
question_answer 25) Intensity at any point due to interference of two waves will be maximum, when path difference at that point is :
A)
\[(2n+1)\,\lambda /2\]
done
clear
B)
\[\text{n}\]
done
clear
C)
\[\]
done
clear
D)
\[\text{/2}\]
done
clear
View Answer play_arrow
question_answer 26) During an experiment, an ideal gas is found to obey an additional law \[V{{P}^{2}}=\] constant. The gas is initially at a temperature T, and volume V. When it expands to a volume 2V, the temperature becomes:
A)
\[\sqrt{2}T\]
done
clear
B)
T
done
clear
C)
4T
done
clear
D)
2T
done
clear
View Answer play_arrow
question_answer 27) A 10 kg body slides down on an inclined plane with a force of 10 N. The minimum force to move up the plane is: (in N)
A)
5
done
clear
B)
10
done
clear
C)
15
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 28) A body starting from rest and travelling with uniform acceleration covers 8 m during the 2nd second. During the 5th second it would travel:
A)
28 m
done
clear
B)
16 m
done
clear
C)
20m
done
clear
D)
24m
done
clear
View Answer play_arrow
question_answer 29) If potential difference across a capacitor is changed from 15 V to 30 V, work done is W. The work done when potential difference is changed from 30 V to 60 V, will be:
A)
W
done
clear
B)
4 W
done
clear
C)
3 W
done
clear
D)
2 W
done
clear
View Answer play_arrow
question_answer 30) There are three wavelengths \[{{10}^{-8}}m,\,\,{{10}^{-2}}m\] and \[{{10}^{8}}m\]. Mark with their respective names from the following:
A)
ultraviolet, microwaves, radio waves
done
clear
B)
visible rays, \[\gamma \]-rays, ultraviolet rays
done
clear
C)
radio waves. X-rays, microwaves
done
clear
D)
X-rays, visible rays, radio waves
done
clear
View Answer play_arrow
question_answer 31) Which one of the following is correct?
A)
Ohm = farad per second
done
clear
B)
Henry = ohm\[\times \]second
done
clear
C)
Henry = farad per second2
done
clear
D)
Farad = ohm per second
done
clear
View Answer play_arrow
question_answer 32) A particle falls from infinity to the earth. Its velocity on reaching earth of radius R is:
A)
\[2Rg\]
done
clear
B)
\[Rg\]
done
clear
C)
\[\sqrt{Rg}\]
done
clear
D)
\[\sqrt{2Rg}\]
done
clear
View Answer play_arrow
question_answer 33) Average number of neutrons emitted per fission is:
A)
1.5
done
clear
B)
2
done
clear
C)
2.5
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 34) For a gas undergoing an adiabatic process, the relation between temperature and volume is found to be \[T{{V}^{0.4}}=\] constant. This gas must be:
A)
Hydrogen
done
clear
B)
argon
done
clear
C)
carbon dioxide
done
clear
D)
helium
done
clear
View Answer play_arrow
question_answer 35) If no heat is lost in condensation of x g of steam at \[100{}^\circ C\]. The ratio of y to x is:
A)
2.5 : 1
done
clear
B)
3 : 1
done
clear
C)
2:1
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 36) The equation of wave travelling in a string can be written as \[y=3\,\cos \,\pi \]\[(100t-x).\] Its wavelength is:
A)
2 cm
done
clear
B)
5 cm
done
clear
C)
100 cm
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 37) You are given a metre scale and a rubber ball. Using this which of the following can you experimentally find?
A)
coefficient of restitution
done
clear
B)
time taken by the ball to strike ground
done
clear
C)
acceleration due to gravity
done
clear
D)
modulus elasticity of rubber
done
clear
View Answer play_arrow
question_answer 38) If there were no atmosphere, the earth would have been:
A)
very hot
done
clear
B)
very cool
done
clear
C)
cooler slightly
done
clear
D)
slightly hotter
done
clear
View Answer play_arrow
question_answer 39) An \[\alpha \]-particle is accelerated to 1 volt. Energy of this particle will be:
A)
16000 eV
done
clear
B)
8000 eV
done
clear
C)
1 eV
done
clear
D)
2 eV
done
clear
View Answer play_arrow
question_answer 40) A ray of light enters from a rarer medium to a denser medium at an angle of incidence i. The reflected and refracted rays make an angle of \[90{}^\circ C\]with each other. The angles of reflection and refraction are r and r respectively. The critical angle of denser medium is:
A)
\[\tan {{\,}^{-1}}(\sin \,i)\]
done
clear
B)
\[\tan {{\,}^{-1}}(\sin \,i)\]
done
clear
C)
\[{{\sin }^{-1}}\,{{(\tan \,i)}^{-1}}\]
done
clear
D)
\[\sin {{\,}^{-1}}(\tan \,r)\]
done
clear
View Answer play_arrow
question_answer 41) Critical mass of a fissionable material can be reduced by:
A)
addition impurities
done
clear
B)
heating
done
clear
C)
cooling
done
clear
D)
surrounding with shield to reflect neutrons
done
clear
View Answer play_arrow
question_answer 42) A galvanometer of resistance \[100\,\Omega \] gives full scale deflection for 20 mV. Find the resistance to be attached so that it gives full scale deflection of 5 V:
A)
\[24.9\times {{10}^{3}}\,\Omega \] in series
done
clear
B)
\[24.9\times {{10}^{3}}\,\Omega \] in parallel
done
clear
C)
\[44.9\times {{10}^{3}}\,\Omega \] in series
done
clear
D)
\[44.9\times {{10}^{3}}\,\Omega \] in parallel
done
clear
View Answer play_arrow
question_answer 43) A nuclear reactor delivers power of 10 W find fuel consumed by the reactor per hour if its efficiency is 20%. Given\[c=3\times {{10}^{8}}m/s\]:
A)
\[2\times {{10}^{-6}}\text{g/hr}\]
done
clear
B)
\[9\times {{10}^{-12}}\text{g/hr}\]
done
clear
C)
\[8\times {{10}^{-9}}\text{g/hr}\]
done
clear
D)
\[2\times {{10}^{-9}}\text{g/hr}\]
done
clear
View Answer play_arrow
question_answer 44) A particle of mass 4 kg is acted upon by steady force of 4 N distance travelled by the particle in 4 sec is:
A)
10m
done
clear
B)
2m
done
clear
C)
4m
done
clear
D)
8 m
done
clear
View Answer play_arrow
question_answer 45) A proton and a-particle are having same kinetic energy. Find the ratio of their linear momentum is:
A)
\[\sqrt{2}:2\]
done
clear
B)
1 : 4
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 46) A particle having angular momentum A. The angular momentum becomes 4 A after 4 seconds. The torque applied is:
A)
A/4
done
clear
B)
\[\frac{3A}{4}\]
done
clear
C)
3A
done
clear
D)
4A
done
clear
View Answer play_arrow
question_answer 47) If temperature is changed from \[27{}^\circ C\] to\[327{}^\circ C\]. The ratio of K.E. molecules at two temperatures:
A)
3 : 1
done
clear
B)
2 : 3
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 48) A wire having resistance \[2\,\Omega .\] If the length of wire is doubled the resistance will be :
A)
\[6\,\Omega \]
done
clear
B)
\[2\,\Omega \]
done
clear
C)
\[8\,\Omega \]
done
clear
D)
\[4\,\Omega \]
done
clear
View Answer play_arrow
question_answer 49) Two equal and opposite charges of \[2\times {{1}^{-10}}C\] are placed at a distance of 1 cm forming a dipole and are placed in a electric field of\[2\times {{10}^{5}}N\text{/}C\]. The maximum torque on dipole is :
A)
\[2\sqrt{2}\times {{10}^{-6}}\text{Nm}\]
done
clear
B)
\[8\times {{10}^{8}}\text{Nm}\]
done
clear
C)
\[4\times {{10}^{-}}^{7}\text{Nm}\]
done
clear
D)
\[4\times {{10}^{-}}^{9}\text{Nm}\]
done
clear
View Answer play_arrow
question_answer 50) Half-life of Ra is 1600 years if initial mass of radium is 400 g. The time after which it decays 100 gm, will be:
A)
6400 years
done
clear
B)
4800 years
done
clear
C)
3200 years
done
clear
D)
1600 years
done
clear
View Answer play_arrow
question_answer 51) The theory of an expanding universe is due to:
A)
Feynmann
done
clear
B)
Eddington
done
clear
C)
Hoyle
done
clear
D)
Hubble
done
clear
View Answer play_arrow
question_answer 52) The mass of a body of the moon is less than on earth. The gravitational force is less in moon than on the earth. Out of the given two statements:
A)
the second statement is false, the first is correct
done
clear
B)
the first statement is false, the second is correct
done
clear
C)
the first is caused and second is result
done
clear
D)
the first statement is result, second is the case
done
clear
View Answer play_arrow
question_answer 53) We have a source of red light and a blue light of same power. Then the red light gives photons per second :
A)
less in number and of less energy
done
clear
B)
more in number and of more energy
done
clear
C)
same in number but of less energy
done
clear
D)
more in number but of less energy
done
clear
View Answer play_arrow
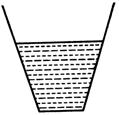
question_answer 54) Two vessels A and B of cross-section as shown in figure contain a liquid upto the same height. As the temperature rises, the liquid pressure at the bottom (neglecting expansion of the vessels) will:
A)
A B increase in B, decrease in A
done
clear
B)
increase in A, decrease in B
done
clear
C)
decrease in both A and B
done
clear
D)
increase in both A and B
done
clear
View Answer play_arrow
question_answer 55) A mass m is lowered with the help of a cord of length d by a disc at a constant acceleration g/4 The work done by the cord will:
A)
\[\frac{3}{4}mgd\]
done
clear
B)
\[\frac{mgd}{8}\]
done
clear
C)
\[\frac{mgd}{4}\]
done
clear
D)
\[mgd\]
done
clear
View Answer play_arrow
question_answer 56) A particle having potential energy 1/3 of the maximum value of a distant of 4 cm from a mean position. The amplitude of the motion is:
A)
\[4\sqrt{3}\]
done
clear
B)
\[6\sqrt{3}\]
done
clear
C)
\[2\sqrt{6}\]
done
clear
D)
\[2/\sqrt{6}\]
done
clear
View Answer play_arrow
question_answer 57) A convex lens of 40 cm focal length is combined with a concave lens of focal length 25 cm. The power of combination is:
A)
- 1.5 D
done
clear
B)
- 6.5 D
done
clear
C)
46.6 D
done
clear
D)
6.6 D
done
clear
View Answer play_arrow
question_answer 58) If radius of the earth is reduced by 1% without changing its mass. The change in g will be:
A)
2% decrease
done
clear
B)
2% increase
done
clear
C)
1% decrease
done
clear
D)
1% increase
done
clear
View Answer play_arrow
question_answer 59) When a particle is rotating in a circle will happen:
A)
no force is acting on particle
done
clear
B)
velocity of particle is constant
done
clear
C)
particle has no acceleration
done
clear
D)
no work is done
done
clear
View Answer play_arrow
question_answer 60) The equivalent capacitance in the system of capacitance will be :
A)
\[1\mu F\]
done
clear
B)
\[2\mu F\]
done
clear
C)
\[1.5\mu F\]
done
clear
D)
\[3\mu F\]
done
clear
View Answer play_arrow
question_answer 61) When glycerol reacts with nitric acid, in presence of cone. \[{{H}_{2}}S{{O}_{4}}\], in cold, it gives:
A)
trinitro glycerine
done
clear
B)
dynamite
done
clear
C)
glycerol trinitrate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 62) Rate of diffusion of gas X is twice the gas V. If molecular weight of X is 8, what is the molecular weight of Y?
A)
32
done
clear
B)
16
done
clear
C)
8
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 63) What is the structure of\[{{H}_{2}}O\]?
A)
Tetrahedral
done
clear
B)
Pyramidal
done
clear
C)
Angular
done
clear
D)
Planar
done
clear
View Answer play_arrow
question_answer 64) Which of the following has smallest size?
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[A{{l}^{3+}}\]
done
clear
D)
\[S{{i}^{4+}}\]
done
clear
View Answer play_arrow
question_answer 65) The ionic product of water increases if:
A)
pressure is decreased
done
clear
B)
\[{{H}^{+}}\] are added
done
clear
C)
\[O{{H}^{-}}\] are added
done
clear
D)
temperature is increased
done
clear
View Answer play_arrow
question_answer 66) An element is in \[{{M}^{3+}}\] form. Its electronic configuration is \[[Ar]\,4{{s}^{1}}\]. What is the atomic number of element?
A)
21
done
clear
B)
19
done
clear
C)
24
done
clear
D)
22
done
clear
View Answer play_arrow
question_answer 67) Green vitriol is:
A)
\[FeS{{O}_{4}}.7\,{{H}_{2}}O\]
done
clear
B)
\[FeS{{O}_{4}}.5\,{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}.5\,{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}.7\,{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 68) Milk is a colloid in which:
A)
a liquid is dispersed in liquid
done
clear
B)
a solid is dispersed in liquid
done
clear
C)
a gas is dispersed in liquid
done
clear
D)
some sugar is dispersed in water
done
clear
View Answer play_arrow
question_answer 69) X-rays are produced due to:
A)
bombarding of electrons on solids
done
clear
B)
bombarding of \[\alpha \text{-}\]particles on solids
done
clear
C)
bombarding of \[\gamma \]-rays on solids
done
clear
D)
bombarding of neutrons on solids
done
clear
View Answer play_arrow
question_answer 70) What is packing fraction of \[{}_{26}F{{e}^{56}}\]? (At. mass = 55.92066)
A)
\[+\,14.167\]
done
clear
B)
\[+\,73.90\]
done
clear
C)
\[-14.167\]
done
clear
D)
\[-\,73.90\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following is not a reducing agent?
A)
\[S{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[Al\]
done
clear
View Answer play_arrow
question_answer 72) Enthalpy of neutralization of \[N{{H}_{4}}OH\]and \[HCl\] is numerically:
A)
57.1 kJ/mol
done
clear
B)
< 57.1 kJ/mol
done
clear
C)
> 57.1 kJ/mol
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 73) Pi-bond (Ti-bond) is formed:
A)
by the overlapping of atomic orbitals on the axis of nuclei
done
clear
B)
by transference of electrons
done
clear
C)
by sidewise overlapping of half-filled p-orbitals
done
clear
D)
by overlapping of s-orbitals with p-orbitals
done
clear
View Answer play_arrow
question_answer 74) Which one of the following molecules, has zero dipole moment?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 75) Which one of the following is an acidic salt?
A)
\[N{{a}_{2}}S\]
done
clear
B)
\[N{{a}_{2}}S{{O}_{3}}\]
done
clear
C)
\[NaHS{{O}_{3}}\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 76) Which is the correct increasing order of ionization energies?
A)
\[N<O<F\]
done
clear
B)
\[F<O<N\]
done
clear
C)
\[N<F<O\]
done
clear
D)
\[O<N<F\]
done
clear
View Answer play_arrow
question_answer 77) Who gave the law of conservation of mass?
A)
Dalton
done
clear
B)
Avogadro
done
clear
C)
Berzilius
done
clear
D)
Hund
done
clear
View Answer play_arrow
question_answer 78) The molecular mass of \[KMn{{O}_{4}}\] is M. The equivalent weight of \[KMn{{O}_{4}}\], when it is converted into \[KMn{{O}_{4}}\] is:
A)
\[M\]
done
clear
B)
\[\frac{M}{3}\]
done
clear
C)
\[\frac{M}{5}\]
done
clear
D)
\[\frac{M}{7}\]
done
clear
View Answer play_arrow
question_answer 79) Azeotropic mixture of \[HCl\] and water has:
A)
\[84%\text{ }HCl\]
done
clear
B)
\[22.2%\text{ }HCl\]
done
clear
C)
\[63%\text{ }HCl\]
done
clear
D)
\[20.2%\text{ }HCl\]
done
clear
View Answer play_arrow
question_answer 80) For a reaction \[2{{N}_{2}}{{O}_{5}}\xrightarrow{{}}4N{{O}_{2}}+{{O}_{2}},\]rate and rate constant are \[1.02\times {{10}^{-4}}\]mol \[{{L}^{-1}}\,{{s}^{-1}}\] and \[3.4\times {{10}^{-5}}\,{{\sec }^{-1}}\]. The concentration of \[{{N}_{2}}{{O}_{5}}\] at that time will be:
A)
\[1.732\,mol\,{{L}^{-1}}\]
done
clear
B)
\[3\,mol\,{{L}^{-1}}\]
done
clear
C)
\[1.02\times {{10}^{-4}}\,mol\,{{L}^{-1}}\]
done
clear
D)
\[3.2\times {{10}^{5}}\,mol\,{{L}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 81) Which is the strongest oxidizing agent?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[L{{i}^{+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 82) Fatty acids are:
A)
unsaturated dicarboxylic acids
done
clear
B)
long chain alkanoic acids
done
clear
C)
aroma tic carboxylic acids
done
clear
D)
aromatic dicarboxylic acids
done
clear
View Answer play_arrow
question_answer 83) The IUPAC name of \[C{{H}_{3}}--\overset{OH}{\mathop{\overset{|}{\mathop{C}}\,}}\,--C{{H}_{2}}--\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{CH}}\,}}\,\cdot CHO\] is:
A)
4-hydroxy-2-methyl pentanal-1
done
clear
B)
2-hyroxy-4-methyl pentanal-1
done
clear
C)
2-methyl pent-4-ol-1-al
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 84) van der Waals gas equation for n mole gas is:
A)
\[\left( P+\frac{a}{V} \right)(V-b)=nRT\]
done
clear
B)
\[\left( P+\frac{na}{V} \right)(V-nb)=nRT\]
done
clear
C)
\[\left( P+\frac{{{n}^{2}}a}{{{V}^{2}}} \right)(V-nb)=nRT\]
done
clear
D)
\[\left( P+\frac{na}{{{V}^{2}}} \right)(V-nb)=nRT\]
done
clear
View Answer play_arrow
question_answer 85) The uncertainity in the momentum of an electron is \[1.0\times {{10}^{-5}}\,kg\,m{{s}^{-1}}\]. The uncertainity in its position will be: \[(h=6.62\times {{10}^{-34}}\,kg\,{{m}^{2}}{{s}^{-1}})\]
A)
\[1.05\times {{10}^{-28}}m\]
done
clear
B)
\[1.05\times {{10}^{-26}}m\]
done
clear
C)
\[5.27\times {{20}^{-30}}m\]
done
clear
D)
\[5.25\times {{10}^{-28}}\,m\]
done
clear
View Answer play_arrow
question_answer 86) \[CH\equiv \equiv CH\xrightarrow[N{{H}_{4}}OH]{AgN{{O}_{3}}}X,\]here \[X\]is:
A)
benzene
done
clear
B)
silver acetalyde
done
clear
C)
cycloctatetraene
done
clear
D)
cycle hexane
done
clear
View Answer play_arrow
question_answer 87) The most reactive hydrocabon is:
A)
ethene
done
clear
B)
ethyne
done
clear
C)
ethane
done
clear
D)
ethyl benzene
done
clear
View Answer play_arrow
question_answer 88) \[CH\equiv \equiv CH\xrightarrow[{{H}_{2}}S{{O}_{4}}]{{{H}_{2}}O/H{{g}^{2+}}}X\xrightarrow{LiAl{{H}_{4}}}Y\xrightarrow{{{P}_{4}}/B{{r}_{2}}}Z,\] Here Z is:
A)
ethylene bromide
done
clear
B)
ethanol
done
clear
C)
ethyl bromide
done
clear
D)
ethylidene bromide
done
clear
View Answer play_arrow
question_answer 89) The most stable carbonium ion among these is:
A)
\[\overset{+}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{6}}{{H}_{5}}}}\,\]
done
clear
B)
\[\overset{+}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}}}\,\]
done
clear
C)
\[\overset{+}{\mathop{C{{H}_{3}}C{{H}_{2}}}}\,\]
done
clear
D)
\[\overset{+}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}}}\,\]
done
clear
View Answer play_arrow
question_answer 90) The chloroform reacts with \[NaOH\] and methyl amine to give:
A)
\[C{{H}_{3}}COONa\]
done
clear
B)
sodium oxalate
done
clear
C)
\[C{{H}_{3}}NC\]
done
clear
D)
\[C{{H}_{3}}CN\]
done
clear
View Answer play_arrow
question_answer 91) How many isomeric forms of \[{{C}_{7}}{{H}_{9}}N\] contain a benzene ring?
A)
4
done
clear
B)
5
done
clear
C)
6
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 92) Which of the following molecule contains asymmetric carbon atom?
A)
\[C{{H}_{3}}CHCl.COOH\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-COOH\]
done
clear
C)
\[Cl\cdot C{{H}_{2}}\cdot C{{H}_{2}}COOH\]
done
clear
D)
\[C{{l}_{2}}CHCOOH\]
done
clear
View Answer play_arrow
question_answer 93) \[C{{H}_{3}}\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,\cdot C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\xrightarrow{C{{H}_{3}}MgBr}A,\]Here A is:
A)
\[C{{H}_{3}}--\underset{OH}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\cdot C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\]
done
clear
B)
\[\underset{O}{\mathop{\underset{||}{\mathop{C{{H}_{3}}CC{{H}_{2}}\cdot C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,}}\,\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 94) \[{{C}_{6}}{{H}_{5}}\cdot C{{H}_{3}}\xrightarrow[{{H}_{2}}S{{O}_{4}}]{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}Y,\]Here \[Y\]is:
A)
benzaldehyde
done
clear
B)
toluene
done
clear
C)
benzoic acid
done
clear
D)
ethyl benzene
done
clear
View Answer play_arrow
question_answer 95) Reaction of ethyne with HCN, in the presence of \[Ba{{(CN)}_{2}}\] is:
A)
electrophillic addition reaction
done
clear
B)
nucleophillic addition reaction
done
clear
C)
free radical addition reaction
done
clear
D)
electrophillic substitution reaction
done
clear
View Answer play_arrow
question_answer 96) Empirical formula of a hydrocarbon containing 80% carbon and 20% hydrogen is:
A)
\[CH\]
done
clear
B)
\[C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 97) The ion that can be precipitated by \[HCl\] as well as \[{{H}_{2}}S\] is:
A)
\[P{{b}^{2+}}\]
done
clear
B)
\[F{{e}^{3+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 98) Producer gas is:
A)
\[CO+{{N}_{2}}\]
done
clear
B)
\[C{{H}_{4}}+{{H}_{2}}+CO+C{{O}_{2}}\]
done
clear
C)
\[CO+{{H}_{2}}+{{N}_{2}}\]
done
clear
D)
\[C{{H}_{4}}+CO+{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) Which of the following is in the increasing order of the ionic character?
A)
\[PbC{{l}_{4}}<PbC{{l}_{2}}<CaC{{l}_{2}}<NaCl\]
done
clear
B)
\[PbC{{l}_{2}}<\text{ }PbC{{l}_{4}}\text{ }<\text{ }CaC{{l}_{2}}\text{ }<\text{ }NaCl\]
done
clear
C)
\[PbC{{l}_{2}}<PbC{{l}_{4}}<NaCl<CaC{{l}_{2}}\]
done
clear
D)
\[PbC{{l}_{4}}<PbC{{l}_{2}}<NaCl<CaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) Which of the following has tetrahedral structure?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[PO_{4}^{3-}\]
done
clear
D)
\[PC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 101) The catalyst used in Ostwalds method nitric acid preparation, is:
A)
platinised asbestos
done
clear
B)
\[{{V}_{2}}{{O}_{5}}\]
done
clear
C)
\[NO\]
done
clear
D)
\[Fe\]
done
clear
View Answer play_arrow
question_answer 102) 20% volume \[{{H}_{2}}{{O}_{2}}\] solution has a strength of about:
A)
30%
done
clear
B)
10%
done
clear
C)
6%
done
clear
D)
3%
done
clear
View Answer play_arrow
question_answer 103) Which complex has square planar structure?
A)
\[[Ni{{(CO)}_{4}}]\]
done
clear
B)
\[{{[NiC{{l}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 104) The principal constituent of pyrex glass is:
A)
Cl
done
clear
B)
Pb
done
clear
C)
B
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 105) When lead nitrate is heated, it gives:
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO\]
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 106) Solid \[PC{{l}_{5}}\] exists as:
A)
\[PC{{l}_{5}}\]
done
clear
B)
\[PCl_{4}^{+}\]
done
clear
C)
\[PCl_{6}^{-}\]
done
clear
D)
\[PCl_{4}^{+}\]and \[PCl_{6}^{-}\]
done
clear
View Answer play_arrow
question_answer 107) The compound, which is the most stable, is:
A)
\[SnC{{l}_{2}}\]
done
clear
B)
\[CC{{l}_{2}}\]
done
clear
C)
\[GeC{{l}_{2}}\]
done
clear
D)
\[PbC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 108) Which one of the following noble gases, is least polarisable?
A)
Helium
done
clear
B)
Xenon
done
clear
C)
Argon
done
clear
D)
Neon
done
clear
View Answer play_arrow
question_answer 109) The substance, which is added to ore in order to remove impurities during smelting, is known as:
A)
catalyst
done
clear
B)
gangue
done
clear
C)
flux
done
clear
D)
slag
done
clear
View Answer play_arrow
question_answer 110) Which of the following is not a drying and dehydrating agent?
A)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
B)
Silica gel
done
clear
C)
Hydrated \[CaC{{l}_{2}}\]
done
clear
D)
Cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 111) Plaster of Pairs is:
A)
\[CaS{{O}_{4}}.\,{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}\]
done
clear
C)
\[CaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
D)
\[2CaS{{O}_{4}}.{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 112) The chemical formula of lunar caustic is:
A)
\[A{{g}_{2}}S\]
done
clear
B)
\[A{{a}_{2}}S{{O}_{4}}\]
done
clear
C)
\[AgN{{O}_{3}}\]
done
clear
D)
\[AgCl\]
done
clear
View Answer play_arrow
question_answer 113) The highest oxidation state shown by Mn is:
A)
+3
done
clear
B)
+2
done
clear
C)
+4
done
clear
D)
+7
done
clear
View Answer play_arrow
question_answer 114) Which of the following represents a chela ting ligand?
A)
\[{{H}_{2}}\overset{.\,\,\,.}{\mathop{O}}\,:\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[DMG\]
done
clear
D)
\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 115) Compared with the alkaline earth metals, the alkali metals exhibit:
A)
smaller ionic radii
done
clear
B)
higher boiling points
done
clear
C)
greater hardness
done
clear
D)
lower ionization energies
done
clear
View Answer play_arrow
question_answer 116) The compounds of alkaline earth metals have the following magnetic nature:
A)
diamagnetic
done
clear
B)
paramagnetic
done
clear
C)
ferromagnetic
done
clear
D)
antiferromagnetic
done
clear
View Answer play_arrow
question_answer 117) Sulphuric acid reacts with \[PC{{l}_{5}}\] to gives:
A)
sulphuryl chloride \[(S{{O}_{2}}C{{l}_{2}})\]
done
clear
B)
\[PC{{l}_{5}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 118) Micro-cosmic salt is:
A)
\[N{{a}_{2}}HP{{O}_{4}}\]
done
clear
B)
\[Na{{(N{{H}_{4}})}_{2}}P{{O}_{4}}\]
done
clear
C)
\[Na(N{{H}_{4}})HP{{O}_{4}}\]
done
clear
D)
\[Na(N{{H}_{4}})\cdot HP{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 119) When a graph is plotted between log K and\[\left( \frac{1}{T} \right),\] the slope of line obtained represents: (K = rate constant, T = temperature)
A)
\[\frac{{{E}_{a}}}{2.303R}\]
done
clear
B)
\[-\frac{{{E}_{a}}}{R}\]
done
clear
C)
\[-\frac{{{E}_{a}}}{2.303R}\]
done
clear
D)
\[\log A\]
done
clear
View Answer play_arrow
question_answer 120) Entropy is highest of:
A)
water
done
clear
B)
steam
done
clear
C)
ice
done
clear
D)
dry ice
done
clear
View Answer play_arrow
question_answer 121) Which of the following is important for speciation?
A)
Seasonal isolation
done
clear
B)
Reproductive isolation
done
clear
C)
Behavioural isolation
done
clear
D)
Temporal isolation
done
clear
View Answer play_arrow
question_answer 122) In a population, unrestricted reproductive capacity is called:
A)
biotic potential
done
clear
B)
fertility
done
clear
C)
carrying capacity
done
clear
D)
birth rate
done
clear
View Answer play_arrow
question_answer 123) Some bacteria are able to grow in Streptomycin containing medium due to:
A)
natural selection
done
clear
B)
induced mutation
done
clear
C)
reproductive isolation
done
clear
D)
genetic drift
done
clear
View Answer play_arrow
question_answer 124) Which of the following are homologous organs?
A)
Wings of birds and locust
done
clear
B)
Wings of birds (Sparrow) and pectoral fins of fish
done
clear
C)
Wings of bat and butterfly
done
clear
D)
Legs of frog and cockroach
done
clear
View Answer play_arrow
question_answer 125) Which is a reducing sugar?
A)
Galactose
done
clear
B)
Gluconic acid
done
clear
C)
\[\beta \]-methyl galactoside
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 126) Reason of fast speciation in present day crop plants is:
A)
mutation
done
clear
B)
isolation
done
clear
C)
polyploidy
done
clear
D)
sexual reproduction
done
clear
View Answer play_arrow
question_answer 127) Cause of mimicry is:
A)
attack (offence)
done
clear
B)
protection (defence)
done
clear
C)
both (a) and (b)
done
clear
D)
isolation
done
clear
View Answer play_arrow
question_answer 128) Protein synthesis in an animal cell, takes place:
A)
only in the cytoplasm
done
clear
B)
in the nucleolus as well as in the cytoplasm
done
clear
C)
in the cytoplasm as well as in mitochondria
done
clear
D)
only on ribosomes attached to nucleus
done
clear
View Answer play_arrow
question_answer 129) Formation of ozone hole is maximum over:
A)
India
done
clear
B)
Antarctica
done
clear
C)
Europe
done
clear
D)
Africa
done
clear
View Answer play_arrow
question_answer 130) Root pressure is maximum when:
A)
transpiration is low and absorbtion is high
done
clear
B)
transpiration is high and absorbtion is low
done
clear
C)
both (a) and (b)
done
clear
D)
both transpiration and absorption are low
done
clear
View Answer play_arrow
question_answer 131) Insectivorous plants grow where soil deficient in:
A)
nitrogen
done
clear
B)
magnesium
done
clear
C)
phosphorus
done
clear
D)
calcium
done
clear
View Answer play_arrow
question_answer 132) Plants growing in acidic soil are termed as:
A)
halophytes
done
clear
B)
oxalophytes
done
clear
C)
acidophytes
done
clear
D)
hydrophytes
done
clear
View Answer play_arrow
question_answer 133) The genetic material in transduction is:
A)
DNA
done
clear
B)
RNA
done
clear
C)
either DNA or RNA
done
clear
D)
both DNA or RNA
done
clear
View Answer play_arrow
question_answer 134) Mitochondria in middle piece of sperm is called:
A)
ring centriole
done
clear
B)
spenniochondria
done
clear
C)
chondrisome
done
clear
D)
myochondria
done
clear
View Answer play_arrow
question_answer 135) Among the following monosomic aneuploid is:
A)
Klinefelters syndrome
done
clear
B)
Edwards syndrome
done
clear
C)
Downs syndrome
done
clear
D)
Turners syndrome
done
clear
View Answer play_arrow
question_answer 136) Root germination is inhibited by:
A)
abscissic add
done
clear
B)
ethylene
done
clear
C)
auxin
done
clear
D)
gibberellin
done
clear
View Answer play_arrow
question_answer 137) Alkaptonuria is:
A)
dominant sex linked
done
clear
B)
dominant autosomal
done
clear
C)
recessive sex limited
done
clear
D)
recessive autosomal
done
clear
View Answer play_arrow
question_answer 138) Which of the following have 9.3 calories energy value?
A)
protein
done
clear
B)
carbohydrate
done
clear
C)
vitamin
done
clear
D)
fat
done
clear
View Answer play_arrow
question_answer 139) A normal woman whose father was colorblind marries a colourblind man. What is the probability of colourblind girl child?
A)
25%
done
clear
B)
50%
done
clear
C)
75%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 140) Study of ant is called:
A)
Myremecology
done
clear
B)
Insectology
done
clear
C)
Ostology
done
clear
D)
Anticology
done
clear
View Answer play_arrow
question_answer 141) Mo is important for plant in:
A)
nitrogen fixation
done
clear
B)
photosynthesis
done
clear
C)
ATP formation
done
clear
D)
photoperiodism
done
clear
View Answer play_arrow
question_answer 142) Pinocytosis and phagocytosis are absent in:
A)
eukaryotic plant cells
done
clear
B)
eukaryotic animal cells
done
clear
C)
prokaryotic cells
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 143) When a heterozygous offspring is crossed with homozygous recessive parent, the cross is known as :
A)
test cross
done
clear
B)
back cross
done
clear
C)
reciprocal cross
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 144) A plasmid is made up of:
A)
f-RNA
done
clear
B)
m-RNA
done
clear
C)
r-RNA
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 145) Monoecious condition is found in:
A)
Pteridium
done
clear
B)
Pinus
done
clear
C)
Selaginella
done
clear
D)
Cycas
done
clear
View Answer play_arrow
question_answer 146) In Spirogyra during the germination of zygospore how many haploid nuclei takes part?
A)
Three
done
clear
B)
Two
done
clear
C)
One
done
clear
D)
All four
done
clear
View Answer play_arrow
question_answer 147) Wheat germ is:
A)
coleoptile
done
clear
B)
cotyledons
done
clear
C)
embryo
done
clear
D)
endosperm
done
clear
View Answer play_arrow
question_answer 148) Seeds of the orchids are:
A)
minute and sticky
done
clear
B)
light and dry
done
clear
C)
large and heavy
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 149) Amphivasal or leptocentric vascular bundles are found in:
A)
Maize and wheat
done
clear
B)
Helianthus and Cucurbita
done
clear
C)
Dracaena and Yacca
done
clear
D)
Cycas and Dryopteris
done
clear
View Answer play_arrow
question_answer 150) Which of the following cells is not totipotent?
A)
Pith cell
done
clear
B)
Epidermal cell
done
clear
C)
Sieve cell
done
clear
D)
Pollen grain
done
clear
View Answer play_arrow
question_answer 151) Which one of the following elements is necessary for the translocation of sugars in plants?
A)
Boron
done
clear
B)
Iron
done
clear
C)
Molybdenum
done
clear
D)
Manganese
done
clear
View Answer play_arrow
question_answer 152) The first product of \[C{{O}_{2}}\] fixation in Hatch and Slack cycle in plants is:
A)
formation of bundle sheath cells
done
clear
B)
formation of oxaloacetate by carboxylation of phosphoenol pyruvate (PEP) in the mesophyll cells
done
clear
C)
formation of phosphoglyceric acid in mesophyll cells
done
clear
D)
formation of oxaloacetate by carboxylation of phosphoenol pyruvate (PEP) in bundle sheath cells
done
clear
View Answer play_arrow
question_answer 153) R. Q. is:
A)
\[\frac{C{{O}_{2}}}{{{O}_{2}}}\]
done
clear
B)
\[\frac{{{O}_{2}}}{C{{O}_{2}}}\]
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 154) In plants, enzymes are present:
A)
only in leaves
done
clear
B)
only in flowers
done
clear
C)
only in storage organs
done
clear
D)
in all the living cells of plant body
done
clear
View Answer play_arrow
question_answer 155) Nitrogen oxide is produced in the exhaust of:
A)
automobile smoke
done
clear
B)
burning of fuel
done
clear
C)
fertilizer plants
done
clear
D)
cigarette smoke
done
clear
View Answer play_arrow
question_answer 156) Dudhwa National Park is located in:
A)
Arunachal Pradesh
done
clear
B)
Madhya Pradesh
done
clear
C)
Uttar Pradesh
done
clear
D)
Himachal Pradesh
done
clear
View Answer play_arrow
question_answer 157) Lymph nodes are concerned with the production of:
A)
urea
done
clear
B)
lutemizing hormone
done
clear
C)
red blood cells
done
clear
D)
germ-killing cells
done
clear
View Answer play_arrow
question_answer 158) Which of the following sets of derivatives of integumentary structures characterized birds as glorified reptiles?
A)
Syrinx and uropygial glands
done
clear
B)
Claws and uropygial glands
done
clear
C)
Scales and claws
done
clear
D)
Syrinx and scales
done
clear
View Answer play_arrow
question_answer 159) The immediate energy source of muscle contraction:
A)
lactic add
done
clear
B)
glycogen
done
clear
C)
ATP
done
clear
D)
phosphor creatine
done
clear
View Answer play_arrow
question_answer 160) The most important function of diaphragm is:
A)
to aid in digestion
done
clear
B)
to aid in respiration
done
clear
C)
to protect lungs
done
clear
D)
to divide body cavity into comparunents
done
clear
View Answer play_arrow
question_answer 161) Pulse can be detected on a superficial artery like that of:
A)
humors
done
clear
B)
diaphragm
done
clear
C)
thigh
done
clear
D)
wrist
done
clear
View Answer play_arrow
question_answer 162) Brain lies in:
A)
cranium
done
clear
B)
vertebral column
done
clear
C)
tympanic bull
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 163) Blind spot in the vertebrate eye is the place where:
A)
the image does not fall
done
clear
B)
there are neither rods nor cones
done
clear
C)
there are no rods
done
clear
D)
there are no cones
done
clear
View Answer play_arrow
question_answer 164) Posterior pituitary contains and releases:
A)
oxytocin, prolactin and GH
done
clear
B)
oxytocin and estrogen
done
clear
C)
vasopressin and estrogen
done
clear
D)
oxytocin and vasopressin
done
clear
View Answer play_arrow
question_answer 165) Cells of Leydig are found in:
A)
kidneys
done
clear
B)
ovary
done
clear
C)
somniferous tubules
done
clear
D)
liver
done
clear
View Answer play_arrow
question_answer 166) In a double stranded DNA molecule the percentage of cytosine is 18. What is the percentage of adenine?
A)
36%
done
clear
B)
18%
done
clear
C)
32%
done
clear
D)
64%
done
clear
View Answer play_arrow
question_answer 167) In human body urea is produced as an excretory product in the:
A)
liver
done
clear
B)
kidneys
done
clear
C)
urinary bladder
done
clear
D)
pancreas
done
clear
View Answer play_arrow
question_answer 168) Tissue present in an annual ring is:
A)
secondary xylem and secondary phloem
done
clear
B)
primary xylem and primary phloem
done
clear
C)
secondary xylem only
done
clear
D)
primary phloem and secondary xylem
done
clear
View Answer play_arrow
question_answer 169) Structure of DNA was given by:
A)
Moorage
done
clear
B)
Mule
done
clear
C)
Watson and Crick
done
clear
D)
Wilkins
done
clear
View Answer play_arrow
question_answer 170) Amniocentesis is:
A)
process to know about the diseases of brain
done
clear
B)
process to determine any hereditary disease in the embryo
done
clear
C)
process to determine any disease in heart
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 171) Change in the sequence of nucleotide in DNA is called as:
A)
mutagen
done
clear
B)
mutation
done
clear
C)
recombination
done
clear
D)
translation
done
clear
View Answer play_arrow
question_answer 172) Which of the following is a correct match?
A)
Downs syndrome- 21st chromosome
done
clear
B)
Sickel cell anaemia- X- chromosome
done
clear
C)
Haemophilia- Y- chromosome
done
clear
D)
Parkinson disease- X and Y chromosome Parkinson disease-X and Y-chromosome
done
clear
View Answer play_arrow
question_answer 173) Which fungal disease spreads by seed and flowers?
A)
Loose smut of wheat
done
clear
B)
Corn smut
done
clear
C)
Covered smut of barley
done
clear
D)
Soft rot of potato
done
clear
View Answer play_arrow
question_answer 174) Sequence of which of the following is used to know the phylogeny?
A)
m-RNA
done
clear
B)
r-RNA
done
clear
C)
t-RNA
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 175) Which of the following does not secrete toxins during storage conditions of crop plants?
A)
Aspergillus
done
clear
B)
Penicillium
done
clear
C)
Fusarium
done
clear
D)
Colletotrichum
done
clear
View Answer play_arrow
question_answer 176) Which of the following plants produces seeds but not flowers?
A)
Maize
done
clear
B)
Mint
done
clear
C)
Peepal
done
clear
D)
Pinus
done
clear
View Answer play_arrow
question_answer 177) Best material for the study of mitosis in laboratory is:
A)
anther
done
clear
B)
root tip
done
clear
C)
leaf tip
done
clear
D)
ovary
done
clear
View Answer play_arrow
question_answer 178) Mitotic spindle is mainly composed of which proteins?
A)
actin
done
clear
B)
myosin
done
clear
C)
actomyosin
done
clear
D)
myoglobin
done
clear
View Answer play_arrow
question_answer 179) Ribosomes are produced in:
A)
nucleolus
done
clear
B)
cytoplasm
done
clear
C)
mitochondria
done
clear
D)
Golgi body
done
clear
View Answer play_arrow
question_answer 180) Which of the following occurs more than one?
A)
Chromatid
done
clear
B)
Chromomere
done
clear
C)
Centromere
done
clear
D)
Telomere
done
clear
View Answer play_arrow
question_answer 181) Directions: Select the word or phrase, which is nearest in meaning to the given word. NEXUS:
A)
a linked group
done
clear
B)
noxious
done
clear
C)
shibboleth
done
clear
D)
a discrete group
done
clear
View Answer play_arrow
question_answer 182) Directions: Select the word or phrase, which is nearest in meaning to the given word. DIABOLIC:
A)
having double meaning
done
clear
B)
angelic
done
clear
C)
fiendish
done
clear
D)
authoritarian
done
clear
View Answer play_arrow
question_answer 183) Directions: Select the word or phrase, which is nearest in meaning to the given word. CARICATURE:
A)
miniature
done
clear
B)
aberration
done
clear
C)
consequential
done
clear
D)
cartoon likeness
done
clear
View Answer play_arrow
question_answer 184) Directions: Select the word or phrase, which is nearest in meaning to the given word. COEVAL:
A)
contemporary
done
clear
B)
extol
done
clear
C)
intrepid
done
clear
D)
subordinate
done
clear
View Answer play_arrow
question_answer 185) Directions: Select the word or phrase, which is nearest in meaning to the given word. UPBRAID:
A)
extol
done
clear
B)
revile
done
clear
C)
restrain
done
clear
D)
sanction
done
clear
View Answer play_arrow
question_answer 186) Directions: In the following questions, a proverb/idiom/phrase is followed by four alternative choices. Select from amongst the given alternatives which best explains the meaning of the proverb/idiom/phrase. One swallow does not always made a summer:
A)
one hot day does not mean that summer has set in
done
clear
B)
one difficulty overcome does not mean that trouble is over
done
clear
C)
one secret cannot save a persons life
done
clear
D)
spring does not always follow a winter
done
clear
View Answer play_arrow
question_answer 187) Directions: In the following questions, a proverb/idiom/phrase is followed by four alternative choices. Select from amongst the given alternatives which best explains the meaning of the proverb/idiom/phrase. Sent to Coventry:
A)
ostracized
done
clear
B)
sent to public school
done
clear
C)
welcomed
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 188) Directions: In the following questions, a proverb/idiom/phrase is followed by four alternative choices. Select from amongst the given alternatives which best explains the meaning of the proverb/idiom/phrase. To have a second string to ones bow:
A)
o have second alternative in case the first one fails
done
clear
B)
to keep ones powder dry
done
clear
C)
to deceive someone
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 189) Directions: In the following questions, a proverb/idiom/phrase is followed by four alternative choices. Select from amongst the given alternatives which best explains the meaning of the proverb/idiom/phrase. To look a gift-horse in the mouth:
A)
to pick flaws in a gift or favour
done
clear
B)
to be indifferent
done
clear
C)
to appreciate a gift or favour
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 190) Directions: In the following questions, a proverb/idiom/phrase is followed by four alternative choices. Select from amongst the given alternatives which best explains the meaning of the proverb/idiom/phrase. To make fish of one and flesh of another:
A)
to be impartial
done
clear
B)
to be extra smart
done
clear
C)
to become a non-vegetarian
done
clear
D)
to be partial
done
clear
View Answer play_arrow
question_answer 191) Directions: In these questions a sentence is given which contain an idiom or an idiomatic expression which has been italicized. Below it, four alternative meanings of the idiom have been suggested. Choose the correct meaning It has been raining cats and dogs:
A)
raining lightly
done
clear
B)
raining musically
done
clear
C)
raining cruelly
done
clear
D)
raining heavily
done
clear
View Answer play_arrow
question_answer 192) Directions: In these questions a sentence is given which contain an idiom or an idiomatic expression which has been italicized. Below it, four alternative meanings of the idiom have been suggested. Choose the correct meaning He was cool as a cucumber:
A)
fainted
done
clear
B)
nervous
done
clear
C)
dead
done
clear
D)
cool & composed
done
clear
View Answer play_arrow
question_answer 193) Directions: In these questions a sentence is given which contain an idiom or an idiomatic expression which has been italicized. Below it, four alternative meanings of the idiom have been suggested. Choose the correct meaning The long and the short of it is that they decided to marry:
A)
staunchly
done
clear
B)
stoutly
done
clear
C)
all that need be left unsaid is
done
clear
D)
all that can need and be said is
done
clear
View Answer play_arrow
question_answer 194) Directions: In these questions a sentence is given which contain an idiom or an idiomatic expression which has been italicized. Below it, four alternative meanings of the idiom have been suggested. Choose the correct meaning He refused point-blank to compromise on principles:
A)
in a soft voice
done
clear
B)
coldly
done
clear
C)
flatly
done
clear
D)
hotly
done
clear
View Answer play_arrow
question_answer 195) Directions: In these questions a sentence is given which contain an idiom or an idiomatic expression which has been italicized. Below it, four alternative meanings of the idiom have been suggested. Choose the correct meaning As our army attacked, the enemy retreated pell-mell:
A)
in an orderly manner
done
clear
B)
in a disorderly manner
done
clear
C)
in a courageous manner
done
clear
D)
in a heap
done
clear
View Answer play_arrow
question_answer 196) Directions: In these questions there are four words in each group. Out of these, one word is miss- spelt, put a cross in your answer-sheet in the circle under the letter (a), (b), (c), (d) against the miss- spelt word.
A)
bemuse
done
clear
B)
benefit
done
clear
C)
benevolent
done
clear
D)
because
done
clear
View Answer play_arrow
question_answer 197) Directions: In these questions there are four words in each group. Out of these, one word is miss- spelt, put a cross in your answer-sheet in the circle under the letter (a), (b), (c), (d) against the miss- spelt word.
A)
venerable
done
clear
B)
vehical
done
clear
C)
veritable
done
clear
D)
fearful
done
clear
View Answer play_arrow
question_answer 198) Directions: In these questions there are four words in each group. Out of these, one word is miss- spelt, put a cross in your answer-sheet in the circle under the letter (a), (b), (c), (d) against the miss- spelt word.
A)
permanent
done
clear
B)
permissible
done
clear
C)
prevalent
done
clear
D)
perennial
done
clear
View Answer play_arrow
question_answer 199) Directions: In these questions there are four words in each group. Out of these, one word is miss- spelt, put a cross in your answer-sheet in the circle under the letter (a), (b), (c), (d) against the miss- spelt word.
A)
envelope
done
clear
B)
enquiry
done
clear
C)
employement
done
clear
D)
worried
done
clear
View Answer play_arrow
question_answer 200) Directions: In these questions there are four words in each group. Out of these, one word is miss- spelt, put a cross in your answer-sheet in the circle under the letter (a), (b), (c), (d) against the miss- spelt word.
A)
wearysome
done
clear
B)
worried
done
clear
C)
weird
done
clear
D)
wanded
done
clear
View Answer play_arrow
 100 N
100 N 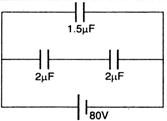 60 \[\,\mu C\]
60 \[\,\mu C\] 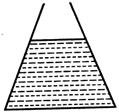
 A B increase in B, decrease in A
A B increase in B, decrease in A 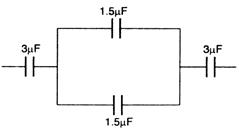 \[1\mu F\]
\[1\mu F\] 