A) 90 J
B) 1 J
C) 99 J
D) 100 J
Correct Answer: A
Solution :
| [a] |
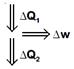 |
| \[\frac{\Delta {{Q}_{1}}-\Delta {{Q}_{2}}}{\Delta {{Q}_{1}}}=\frac{1}{10}=\frac{\Delta w}{\Delta {{Q}_{1}}}\] |
| \[\Rightarrow \frac{1}{10}=\frac{\Delta w}{\Delta {{Q}_{1}}}\] |
| \[\therefore \,\,\,\,\,\,\,\,\Delta {{Q}_{1}}=\Delta w\times 10=100\,\,J\] |
| So, \[\Delta {{Q}_{1}}-\Delta {{Q}_{2}}=\Delta w\] |
| \[\Rightarrow \,\,\,\,\,\,100-10=\Delta {{Q}_{2}}=90\,\,J\] |
You need to login to perform this action.
You will be redirected in
3 sec
