| 'n' moles of an ideal gas undergoes a process \[A\to B\]as shown in the figure. The maximum temperature of the gas during the process will be: [JEE MAIN - I 3-4-2016] |
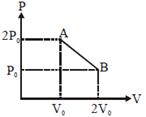 |
A) \[\frac{9{{P}_{0}}{{V}_{0}}}{nR}\]
B) \[\frac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
C) \[\frac{3{{P}_{0}}{{V}_{0}}}{2nR}\]
D) \[\frac{9{{P}_{0}}{{V}_{0}}}{2nR}\]
Correct Answer: B
Solution :
| [b] T will be max where product of PV is max. |
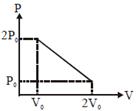 |
| equation of line |
| |
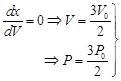 here PV product is max. here PV product is max. |
| |
| Alternate |
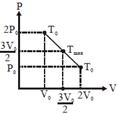 |
| Since initial and final temperature are equal hence maximum temperature is at middle of line. PV = nRT |
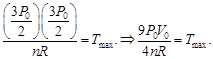 |
You need to login to perform this action.
You will be redirected in
3 sec
