| n moles of an ideal gas undergo a process \[A\to B\]as shown in the figure. Maximum temperature of the gas during the process is [JEE ONLINE 12-05-2012] |
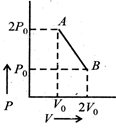 |
A) \[\frac{8{{P}_{0}}{{V}_{0}}}{nR}\]
B) \[\frac{3{{P}_{0}}{{V}_{0}}}{2nR}\]
C) \[\frac{9{{P}_{0}}{{V}_{0}}}{2nR}\]
D) \[\frac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
Correct Answer: B
Solution :
| [b] Work done during the process |
| = Area of trapezium (= area bounded by indicator diagram with F-axis) |
| |
| Ideal gas eqn : PV= nRT |
| |
You need to login to perform this action.
You will be redirected in
3 sec
