A) 3/2
B) 23/15
C) 35/23
D) 4/3
Correct Answer: A
Solution :
| [a] Mayor's formula is |
| and |
| Therefore, using above two relations, we find |
| |
| For monoatomic gas; |
| |
| For diatomic gas; |
| |
| When these two moles are mixed, then heat required to raise the temperature to |
| |
| Hence, for one mole, heat required |
| |
| |
| |
| |
| Alternate Solution |
| As, |
| Here, |
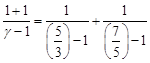 |
| |
| |
| |
| |
| Hence, |
You need to login to perform this action.
You will be redirected in
3 sec
